Wandelt, Kennedy
Bioreactor Design for Viable Recellularization of Whole Porcine Kidney
Faculty Mentor: Dr. Alonzo Cook, Chemical Engineering
Kidney failure affects tens of thousands of individuals annually causing many to rely on dialysis to replace this organ’s function. Most of these individuals are on a waiting list to receive a transplant for years, and unfortunately many die before they are able to reach the top of the list. The bodies of those who do receive kidneys often reject the organs. This rejection can have devastating defects, including mortality. In an effort to change this ongoing cycle we have been researching the idea of creating organs synthesized with the DNA of each specific patient. The process would significantly increase availability of transplant ready organs, and minimize the probability of organ rejection occurrences.
The outline of the process is as follows. First we obtain kidneys from pigs which are comparable in size and structure to human kidneys. Next we decellularize the kidneys, removing the porcine DNA from the structure called the extracellular membrane (ECM) which is made of strictly structural components. Following decellularization is recellularization. This is the process of seeding the ECM with human epithelial cells. To do so we used the bioreactor for which the orca was proposed.
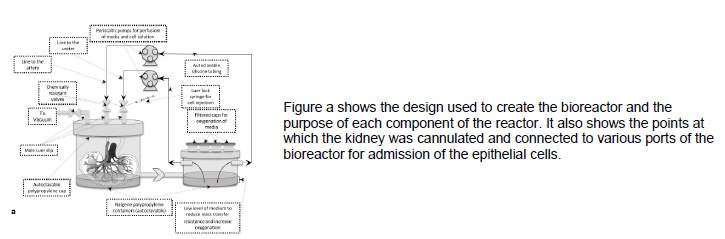
The bioreactor was made with specifications to meet the needs of the recellularization process. It consists of a two chamber design. One chamber to contain the kidney (1000 mL), and another to infuse oxygen into the media (500 mL). The smaller is necessary to maintain a viable environment for the sensitive cells. Sterilization was ensured by superimposing filter caps in the lid of this chamber, these filters allow oxygen to dissolve into the media without allowing particulates or contaminations to do so. The chamber which holds the kidney has three different lines. Two which attach each to the artery and ureter for the perfusion of cells, and one for a vacuum to reduce a positive pressure. Each line is attached to a pump. The artery and ureter lines are fixed to peristaltic pumps for maximum control, while the vacuum line is fastened to a vacuum pump. The peristaltic pumps produce a cycle of media oxygenation and perfusion through the kidney.
My design had four requirements to fulfil. It had to be autoclavable, it had to allow for oxygenation, it had to be large enough to host the kidney yet small enough to be contained in an incubator, and it had to allow easy access for injection and sampling of the cell infused media. Oxygenation was solved simply by the filter caps (as described previously).
Materials: The material chosen to make the bioreactor had to be able to withstand the heat and pressure of the autoclave as this is our chosen method of decontamination. Originally it was intended to use glass, however this would have required tools or glass blowing that were not available. Therefore, polymethylpentene was selected due to its extremely high melting point, low reactivity, and nonporous structure. Though not necessary, the transparent quality of the material is ideal for detection of problems with the cannulation or diffusion mechanisms. For cannulation adaptors polypropylene plastic was used, which has many similar qualities to that of polymethylpentene with the exception of the clarity. The tubing employed for the purpose of kidney to reactor connections is nylon, while the tubes between reactor chambers (and reactor-vacuum tubes) are silicone. In order to secure adaptors and filter caps to the chambers and lids of chambers, Epoxy glue was used. Specifically, the Loctite Stik’n Seal epoxy for outdoor use. This product is used for its durability as well as its efficiency. The bioreactor with these specifications allows for an efficient, sterile, and durable design that can withstand repeated autoclaving and recellularization experiments.
Dimensions: The 1000 mL and 500 mL Nalgene jars used were chosen for their materials as well as the dimensions. Though not as optimized as originally intended, the Nalgene jars were chosen for their ability to fulfil the needs of the bioreactor, as well as for their convenience in price. The 1000 mL chamber, which contained the kidney, was large enough to easily host the organ and could fit within the incubator. The 500 mL chamber was large enough to contain cell infused media enough for two days, while having a wide enough surface area to appropriately oxygenate the media. As a whole these dimensions were ideal for restraints of the incubator and kidney volumes.
Accessibility: Throughout the recellularization process, access is necessary for the sampling and injection of media. To make this possible, the valves used when joining the silicone tubing from peristaltic pump to the lid of the large chamber, were three way allowing the media to flow to a Leur lock syringe when necessary.
The bioreactor created was able to be cost efficient while effectively satisfying the four requirements necessary for the recellularization process. Though the exact dimensions and materials from the preliminary design were not employed, the alternatives are able to sufficiently fulfil the same role. Recellularization research can commence with this optimized design that ensures sterility, accessibility, and feasibility.