Ludlam, Grant
Determining the Structure of the BBSome
Faculty Mentor: Barry Willardson, Department of Chemistry/Biochemistry
Introduction
Bardet-Biedl Syndrome (BBS) occurs when an eight-protein complex called the BBSome is improperly assembled and can no longer function. The BBSome is responsible for trafficking membrane proteins to cilia, a projection from the cell surface packed with receptors that allow the cell to sense its surroundings. Loss of BBSome function causes malformation of cilia and leads to vision loss, obesity, kidney disease and mental retardation.
Little is known about how the BBSome is assembled or how it functions. Our lab is undertaking a structural analysis of the BBSome and several intermediates in the assembly process to understand how the BBSome is put together and how it works. This assembly information can be used to identify how BBSome assembly is disrupted by mutations that cause BBS, allowing pharmaceutical companies to develop therapies that will help restore proper BBSome function in BBS and related diseases involving the cilia.
To complete our picture of BBSome assembly and understand BBSome function, we will need to use advanced biochemical techniques such as cryo-electron microscopy (cryo-EM) and mass spectrometry to find the structure of the BBSome in its final, completed form. These techniques require significant amounts of purified protein. Here, we demonstrate a reliable procedure for purifying large amounts of BBSome complex.
Methods
We purified the BBSome complex using a two-step tandem affinity chromatography approach. First, we purified a known binding partner of the BBSome called BBS3. We created a recombinant version of BBS3 in a pET-DEST42 expression vector. pET-DEST42 is a plasmid designed to express proteins in bacterial cells when induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG). The BBS3 construct also had a triple Flag affinity tag on the end of the protein that we used to separate it from the rest of the proteins in the cell. The first 15 amino acids of BBS3 were deleted to increase the solubility of the protein. The mutation Q73L was introduced to eliminate the GTPase activity of BBS3, locking it in a BBSome binding conformation. DE3 cells where transformed with the BBS3-pDEST42-3xFlag vector and grown to an optical density of 0.4 at 600 nm. The cells were then induced with IPTG for 3 hours. The cells were then centrifuged into a pellet and the supernatant was removed. One gram of pelleted cells where then lysed in Bacterial Protein Extraction Reagent (B-PER) and passed over a Flag antibody resin packed column. The Flag-tagged BBS3 proteins was retained in the column while the rest of the cell contents were washed away.
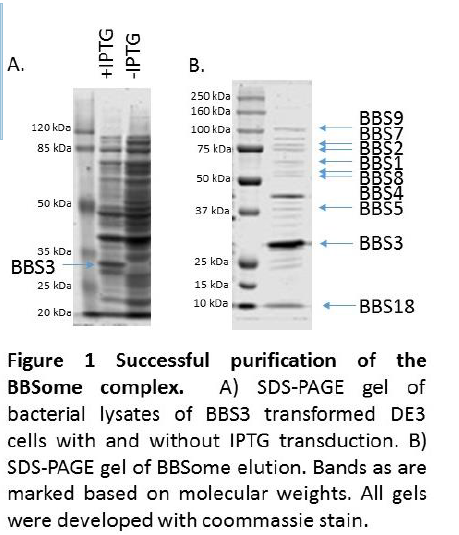
We then purified the BBSome complex from bovine photoreceptor cells by loading a photoreceptor extract onto the BBS3 flag-tagged resin. The BBSome present in the photoreceptors bound to the immobilized BBS3 on the column. The column was washed and the BBSome complex was eluted with a high salt buffer to interrupt the BBSome/BBS3 interaction. The elution was concentrated and frozen and stored at -80 ° C. A sample of the BBS3 elution and the BBSome elution were loaded onto SDS-PAGE gels and analyzed by Coommassie stain.
Results
Analysis of the SDS-PAGE gels of the BBS3 transformed cells showed the presence of a 25 kDa band present in IPTG induced cells but absent in cells that were not induced, indicating that the bacteria successfully expressed the BBS3 construct (Fig 1A). Analysis of the SDS-PAGE gels of the BBSome elution revealed bands corresponding in molecular weight to each of the eight BBSome subunits (Fig 1B).
Our purification procedure yields protein complexes in sufficient purity of cryo-EM analysis. We will send the purified BBSome to our collaborator Dr. Jose Valpuesta in Madrid, Spain for cryo-EM analysis. The Valpuesta lab has recognized expertise in the field of cryo-EM and our lab has a long-standing collaboration with them and have determined the structure of several protein complexes.
In our lab, we will perform chemical crosslinking experiments that can be used to verify and refine the cryo-EM structure from the Valpuesta lab. The crosslinking experiments will give distance constraints between portions of the BBSome and will help us fit the BBSome subunits into the reconstruction from the cryo-EM data.
Conclusion
After we have obtained enough cryo-EM data, we will be able to create a 3D reconstruction of the complex. A high-resolution 3D image will allow us to accurately dock the individual BBSome subunits together and identify how they interact. The data from these experiments will allow drug developers to target interactions between subunits in the BBSome complex. They will be able to use the points of contact to develop therapies to remedy the deleterious mutations present in Bardet-Biedl Syndrome.
1. Zhang, Q., Yu, D., Seo, S., Stone, E. M., and Sheffield, V.C. (2012) Intrinsic protein-protein interaction-mediated and chaperonin-assisted sequential assembly of stable bardet-biedl syndrome protein complex, the BBSome. J Biol Chem 287, 20625-35.
2. Liew, G. M., Ye, F., Nager, A. R., Murphy, J.P., Lee, J. S., Aguiar, M., Breslow, D. K., Gygi, S. P., and Nachury, M.V. (2014) The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell 31, 265-78.
3. Mourão, A., Nager, A. R., Nachury, M. V., and Lorentzen, E. (2010) Structural basis for membrane targeting of the BBSome by ARL6. Nat Struct Mol Biol 21, 1035-41.
4. Seo, S., Baye, L.M., Schulz, N.P., Beck, J.S., Zhang, Q., Slusarski, D. C., and Sheffield, V.C. (2010) BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci, 107, 1488-93.