Luo, Shi
The total synthesis of yaku’amide A
Faculty Mentor: Steven Castle, Department of Chemistry and Biochemistry
Yaku’amide A (YA) has potent toxicity against cancer cells (IC50 = 14 ng/ml
against P388 murine leukemia cells). Ueoka et al.1 first reported the isolation of YA from
a marine sponge in the East China Sea. They used spectroscopy and chemical
degradations to analyze its structure. YA contains unique dehydroamino acids (See
Figure 1). In the growth inhibition study, YA showed unique activity against 39 cancer
cell lines, indicating a novel mode of action. The unusual structure of YA and its
prominent biological activity has attracted research groups from around the world to
address its synthesis.
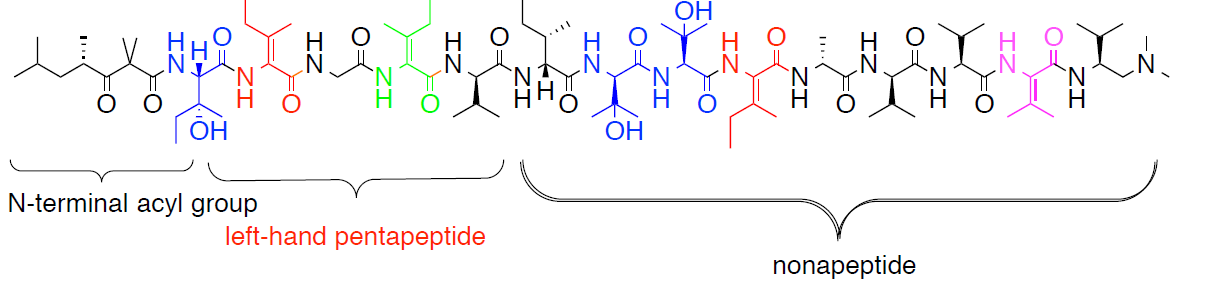
Figure 1. The structure of yaku’amide A.
The linear structure of yaku’amide A contains an N- and C-terminal subunits.
These N- and C-terminal structures are not found in other natural products.1 Besides
Val, the left-hand pentapeptide (Figure 1) also contains E- and Z-ΔIle and (2S,3R)-β-
OHIle. The nonapeptide contains D- and L-β-OHVal. The study and synthesis of these
unusual structures will require the development of new synthetic methodology. The total
synthesis of YA will also be able to provide an abundant supply of YA to allow future
study of its biological activity.
YA demonstrates potent toxicity against different cancer cell lines. Its IC50 value
is 14 ng/ml against P388 leukemia cells.1 YA is potent against breast, colon,
gastrointestinal, and lung cancer cell lines. It has a unique pattern of activity against a
panel of 39 cancer cell lines, which sets it apart from all other 38 anticancer drugs that
have been tested in this panel.1 This indicates that YA has a different mechanism of
inhibition against cancer cells. Our goal is to identify its mode of action.
During the ORCA grant period, I was able to make the necessary amino acid (β-
OHIle) to assemble the left-hand pentapeptide. β-OHIle is accessed by applying aminohydroxylation
reaction with the aid of a chiral auxiliary.
We expected to complete the whole synthesis within this year so that we can
continue to study its biological activity.
1. Ueoka, R.; Ise, Y.; Ohtsuka, S.; Okada, S.; Yamori, T.; Matsunaga, S. Yaku’amides A and B, Cytotoxic Linear Peptides Rich in Dehydroamino Acids from the Marine Sponge Ceratopsion sp. J. Am. Chem. Soc. 2010, 132, 17692–17694.