Hobson, Amanda
Is HDAC1 mediated β-cell proliferation dependent on decreased p15 expression?
Faculty Mentor: Jeffrey Tessem, Nutrition, Dietetics, and Food Science
Introduction
The body maintains normoglycemia through pancreatic β-cells which sense elevation in
circulating glucose levels and secrete insulin to maintain the correct blood glucose
concentration. A decrease in functional pancreatic β-cell mass leads to Type 1 or Type 2
diabetes. Each form has different initial causes, but both forms ultimately result in decreased
functional β-cell mass. Functional β-cell mass is defined as the rate of glucose stimulated insulin
secretion multiplied by the total β-cell mass. The total β-cell mass is a function of cellular
proliferation and death rates. β-cells have a very low proliferation rate, therefore determining
the mechanisms that induce β-cell replication for pancreatic islet transplantation, or increasing
the endogenous β-cell population could be used to cure diabetes.
Nkx6.1 overexpression increases functional β-cell mass (1). We have shown that Nkx6.1
induces AURKA (2), and HDAC1. We have also shown that HDAC1 overexpression induces β-cell
proliferation, maintains glucose stimulated insulin secretion and protects against apoptosis.
HDACs function by removing acetyl groups from histones, which results in decreased expression
of genes. HDACs have been shown to downregulate expression of cell cycle inhibitors. We
hypothesized that HDAC1 mediated proliferation would correspond with decreased expression
of cell cycle inhibitors (CDKI’s). We measured expression of all known CDKI’s, and were able to
show that p15 mRNA was significantly decreased in response to HDAC1 overexpression. The
purpose of this study is to determine the role of p15 in the HDAC1 β-cell proliferation pathway.
Methodology
For the experiments conducted testing the effect of p15 on ß-cell proliferation, we used
the INS-1 832-3 rat insulinoma ß-cell cell line. Cells were transfected with NV (negative control),
GFP (viral control), and HDAC1 (positive control). Twenty-four hours after viral transfection,
cells were exposed to either a siCTRL or sip15. Cell viability was measured in each condition by
cell counts.
For the second set of experiments, we used the same samples from the cell viability
experiment above to measure RNA levels. We harvested for cDNA and then for mRNA and ran a
rtPCR test on each condition. This was to confirm the results from the cell viability experiment.
The third set of experiments was done using the same INS-1 832-3 rat insulinoma ß-cell
cell line. We transfected the cells with NV (negative control), GFP (viral control), or HDAC1
(positive control). Protein samples were collected in time increments of 24, 48, 72, and 96
hours after transfection. The protein samples were then run on a western blot and measured
for p15 protein levels. The ratio of p15 to tubulin proteins were quantified.
Results
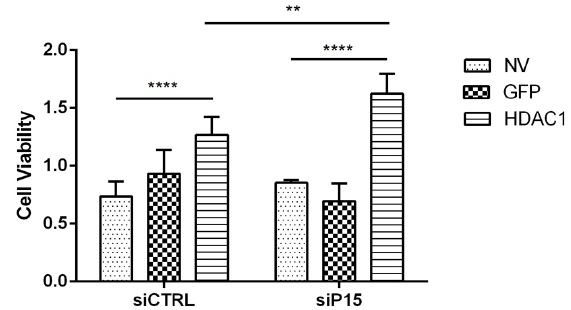
Our results from the first set of experiments measured cell viability after exposing cells to
HDAC1 and sip15. We saw that compared to the NV condition, HDAC1 increased the cell
viability with or without sip15. We also saw that between siCTRL and sip15 HDAC1 conditions that
knocking out p15 significantly increases HDAC1 in its ability to drive cell proliferation.
The results from the second experiment were measuring the amount of RNA in each condition from
the cell viability experiment above. However, the RNA samples collected from this experiment
were insufficient to produce reliable results. Due to time constrictions, I was not able to redo
the entire experiment to get accurate results.
The third experiment measured the ratio of p15 protein to tubulin protein in cells that
overexpressing HDAC1. The majority of the western blots ran measuring the p15 and tubulin
levels had background interference making it difficult to draw conclusive data. Countless
strategies and consultations with professors were discussed to try to solve the problem, but
again due to time constraints there was no time to collect more accurate data. The graphs
included show the preliminary data collected from the western blots.
Discussion and Conclusion
Our hypothesis was confirmed with the results from the sip15 experiment. We saw that
compared to the siCTRL, knocking down p15 (sip15) with HDAC1 overexpression drives the
ability of HDAC1 to promote ß-cell proliferation. Even though the other two experiments had
inconclusive results, we have since researched new lab techniques and procedures that will
help us to collect accurate data with these experiments in the future.
Scholarly Sources
1. J. C. Schisler et al., Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain
transcription factor Nkx6.1. Molecular and cellular biology 28, 3465 (May, 2008).
2. A. Hobson et al., Aurora Kinase A is critical for the Nkx6.1 mediated β-cell proliferation pathway. Islets 7, 1 (June,
2015).