Cary Tippets and Dr. Robert Davis, Physics and Astronomy
Photovoltaics, solar cells, have great potential as an energy source. Currently solar energy is too expensive for it to make a considerable impact on the energy market. Including production and maintenance cost, solar energy is nearly five times more expensive than traditional energy sources such as coal¹. Efficiency and production costs are two aspects contributing to the cost per watt of photovoltaics. If efficiency increases or production cost decreases then the cost per watt will decrease. There are likewise two aspects of production that contribute to its cost: the cost of the absorption material, and the process by which the absorption layer is deposited. In my research, I tested copper oxide as the absorption material and spray pyrolsis for the material deposition in attempt to address both issues influencing production costs.
Copper oxide (CuO) has been examined as a possible candidate for photovoltaic devices due to its affordability and photo-electric properties. An important property of photovoltaic material is its ability to have electrons jump from the valance band to the conduction band. The energy required to excite an electron from the valance band to the conduction band is known as the band gap. CuO has been shown to have a direct band gap of 2.1 eV and an indirect band gap at 1.4 eV². This falls right in the optimal band gap range of 1.4-2.1 eV. As an oxide, CuO can be deposited in an oxygen rich environment without any adverse effect to its chemical make up. This allows the use of spray pyrolysis for the deposition technique.
In this experiment, thin films of CuO were deposited on heated glass and silicon substrates with varying temperatures through an air atomizing nozzle. The pressure of the air supply was held constant at 40 PSI. The nitrate solution was created by mixing a .1 M solution of Cu(NO3)2 H2O dissolved in distilled water. The solution was constantly stirred on a magnetic stir plate to make sure the solution remained dissolved during the process. The solution was siphoned through tubing to the nozzle where it was sprayed onto a heated substrate In this research, the Standing Electron Microscope (SEM), Atomic Force Microscope (AFM) and X-ray photoelectron spectroscopy (XPS) were used to characterize the material deposited. SEM was used to image the surface of the material to visually inspect the surface for defects and thickness. AFM was used to quantitatively verify the roughness of the material. XPS was used to measure the quality and purity of the material and verify that the composition is CuO.
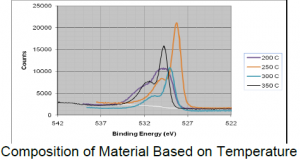
Each sample was deposited at different temperatures between 250° C to 400° C. The optimal temperature to get the purest layer of CuO was found to be 320°C. Cu2O dominated the material at lower temperatures. As the temperature increased, the quantity of CuO increased until ≈300°C was reached, at which point, Cu2O began to dominate the material again. In the experiment, 320°C was found to provide the best result to have the purist layer of CuO. The figure shows the XPS result of the samples’ composition dependant on the temperature. Based on the location of the peaks, we can determine the composition of the material.
The roughness of the material is important because the surface quality affects the scattering of light and the ability of photons to get into the material and absorb. SEM images were taken to visually verify the surface conditions over a larger region of space. Although the smoothness did vary from sample to sample, no correlation was found between the temperature of the deposition and the apparent smoothness of the thin film. Further investigations will continue to help determine the cause of the roughness. Evenly distributed throughout the sample are large particles varying from 0.5 – 1.5 μm in diameter. The cause of these particles is most likely due to a buildup of solids in the nozzle that are then discharged during the spraying process.
CuO samples were placed under a solar simulating light source, while connected to a four prong measurement apparatus, to determine if resistivity would change when placed under light. This measurement would help determine the possible efficiency of a device made from CuO. During this test none of the samples showed a decrease in resistivity, indicating that CuO would not make an effective absorption material.
Although CuO showed no change in resistivity when placed under solar simulation, further tests need to be performed in order to determine if CuO could be used as an absorption material. Investigation of possible doping of CuO could lead to its use in solar cells.

