Lyons, Kyle
EFFECTS OF MUSCLE COOLING ON AMPK AND PROTEIN SYNTHESIS IN SKELETAL MUSCLE
Faculty Mentor: David Thomson, PDBIO
Background
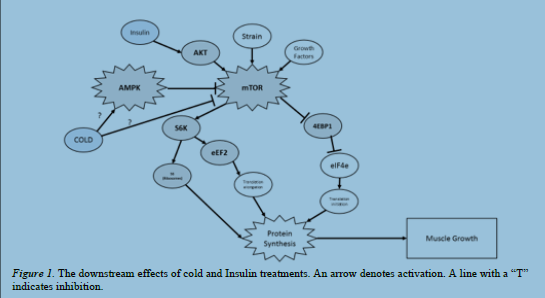
AMP-activated protein kinase (AMPK) is an intracellular protein that regulates many signaling
responses including the mechanistic target of rapamycin (mTOR) pathway, which increases
protein synthesis1. Recent research suggests that cold-water immersion of the leg activates
AMPK in skeletal muscle. It is not known whether cold directly activates AMPK in skeletal
muscle or if the aforementioned results are due to systemic or hormonal responses. Furthermore,
the direct effect of cooling on protein synthesis-related signaling in skeletal muscle has not been
established. If cooling affects the activation of AMPK, then it could also affect mTOR.
Therefore, the purpose of this study was to understand the effect of cooling (COLD) on the
AMPK and mTOR pathways and whether these effects are dependent on AMPK. We
hypothesized that COLD treatment would increase AMPK activation and decrease the activation
of mTOR and its downstream targets. To test this, we used cell culture and in vitro whole muscle
incubation models.
Procedure
C2C12 myoblasts (immature mouse skeletal muscle cells) were cultured in 6-well plates
at 37°C in growth until confluent (covering the bottom of the culture dish). They were then
differentiated to myotubes (mature muscle cells) for approximately 7 days. Once differentiated,
we incubated the cells in serum-free media for 4 hours at 37°C. Compound C (an AMPK
inhibitor) was added to half of the cells ½ hour before the end of this pre-incubation period. At
the end of 4 hours, Puromycin was added to all of the cells (Puromycin will label newly
synthesized proteins) just before incubation. Half of the cells continued incubation at 37ºC and
the other half at 27ºC for 15 minutes. Insulin (100 nM), which stimulates Akt and mTOR
activity, was added to some of the cells after 15 minutes and then the treys were placed back into
incubation for 15 minutes more (30 min total). One trial consisted of two 6-well plates (one for
27°C, the other for 37°C). On each plate there was two control (ctrl) wells, two wells treated
only with insulin, and two wells treated with insulin + compound C (IC). There were three trials
which resulted in three treatment groups (n=6)- Control (ctrl), insulin, and insulin + compound C
(IC). After treatment, cells were lysed in homogenization buffer, freeze-thawed once at -90°C,
and centrifuged. The supernatant was removed and stored for later use at 90°C.
For the muscle incubation experiments, we used muscle from mice (n=10) that have been
genetically altered so that they do not express LKB1 in their skeletal muscle (KO), and from
littermate controls (WT). After euthanization, the Extensor digitorum longus (EDL) (glycolytic,
type 2, fast twitch (white) muscle fiber) and soleus (SOL) (oxidative, type 1, slow twitch/red
fibers) muscles were removed from both hind limbs. Muscles were equilibrated in oxygenated
Dulbecco’s Modified Eagle Medium (DMEM) at 37ºC for 30 min. After the initial incubation
period, one of the muscles was transferred to a tube with fresh, oxygenated DMEM at 37ºC, and
the other was transferred to a tube with fresh, oxygenated DMEM at 27ºC. The muscles were incubated for an additional 15 minutes then blotted dry and frozen in liquid nitrogen. All muscles
were stored in a -90C freezer until prepared for use in western blots. After treatment, the protein quantities of the samples was quantified and loading samples
were prepared for use in western blots.
Results
Exposure of C2C12 myotubes to 27°C temperature (compared to 37°C control) for 15
minutes resulted in decreased phosphorylation of P70-S6 Kinase 1 (S6k) and ribosomal protein
s6 (S6), both of which lie downstream of mTOR signaling, and increased phosphorylation of
eukaryotic translation elongation factor 2 (eEF2). All of the mentioned findings are consistent
with our observation of decreased protein synthesis. However, AMPK was not activated by
COLD, but was inactivated by exposure to Compound C (an AMPK inhibitor). In vitro
incubation of extensor digitorum longus (EDL) muscles (n=10 muscles/group) at 27°C did not
affect the phosphorylation of any proteins except for acetyl-CoA carboxylase (ACC) where we
saw an increase after COLD treatment. Incubation of Soleus (SOL) muscles had no effect accept
for an AMPK-independent decrease in eEF2 activity. Our results suggest that cold may directly
decrease protein synthesis, but AMPK likely does not play a major role. Further research is
needed to determine the applicability of our findings to the use of cryotherapy in the treatment of
muscle injury or soreness.
Outcomes
The results of this project have been presented in a variety of forms and settings. A list of these
forms and settings follows:
• BYU honors thesis
• BYU honors poster presentation
• South-west American College of Sports Medicine (SWACSM) annual conference