Struk, Jeremy
Men’s Hearts Shall Fail Them: A Solution to the Problems of Heart Transplantation
Faculty Mentor: Alonzo Cook, Chemical Engineering
Ischemic Heart disease, according to the World Health Organization, is the leading cause of death in the USA and abroad.1 As a result of the high incidence of the disease and low transplant availability, the field of tissue engineering offers a promising solution. With the new developments in the field, many exciting treatments are available to help stem the tide of the disease, however; there is still a great need for more viable heart replacements to help patients with end stage heart failure. Tissue engineering offers the solution of creating hearts for patients in need that are constructed using the patient’s own stem cells. This could potentially solve the two main problems that heart transplantation therapy faces today: scarcity of transplantable organs and the recipient’s immune system rejecting the transplanted organ.
The research team I work with in Dr. Cook’s lab studies the possibility of creating new hearts for transplantation by decellularizing a porcine heart, and then recellularizing the Extra Cellular Matrix (ECM) with human cells derived from the patient. We use a porcine heart as the source for our ECM because other ECM’s that are produced synthetically don’t have the inherent vasculature and strength as the ECM created during the embryonic and growth phases of a mammal. The team has done a good job creating a decellularization protocol, but now the difficulty for future research is in creating a viable organ for transplantation by reseeding the ECM with various types of cells stemming from the patient.
For my ORCA project, I used and modified my previous casting protocol in order to create better casts of the vasculature of native and decellularized porcine hearts. The first part of the casting process is to decide on a resin. Our resin needed to have the proper viscosity in order to successfully be pumped into the vasculature of the heart. It also needed to be resistant to water and to solutions with very high pH after the hardening was complete. We tested a fiberglass resin that had all these properties as well as variable viscosity when mixed with different amounts of acetone. The resin also held up well to the harsh NaOH solution we used to eventually dissolve the tissue.
After a batch of resin was prepared by mixing in acetone and hardener, we used a 3D printed Hickman still, tubing, and a peristaltic pump or syringe to inject the resin into the aorta of the heart. Prior to the injection, in an attempt to avoid any resin accumulating in the left ventricle of the heart, the aortic valve was sutured, and in some attempts even closed with neodymium magnets on either side of the valve. Despite our attempts to prevent resin from entering into the ventricle, the resin always found its way inside, only to form a large cast of the interior of the left ventricle. We noticed that the thick piece of resin gave inherent stability and structure to the rest of the casting and eventually ceased our attempts to create a casting void of a left ventricle. With a stronger cast, this allowed us to focus our efforts on a tissue degradation technique that was faster and less damaging to the cast than the previous techniques used.
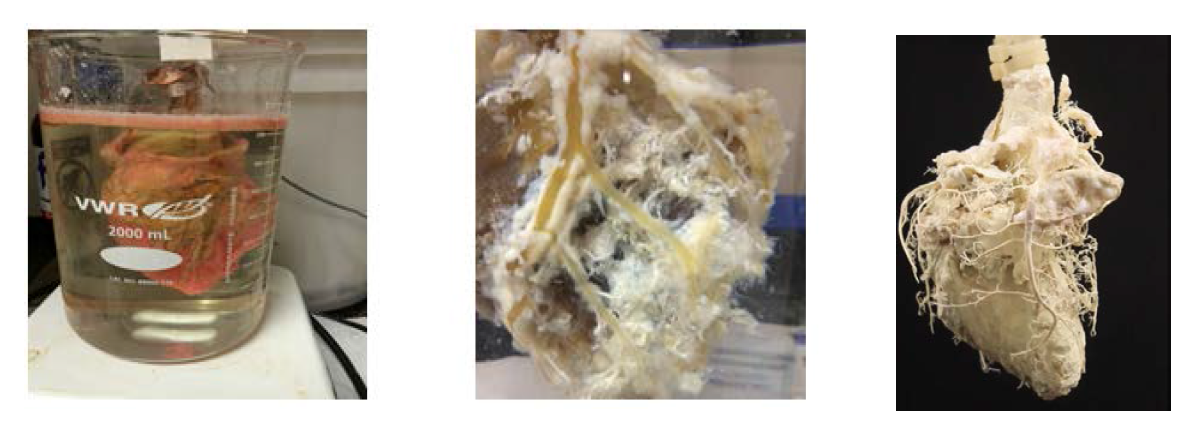
We discovered that as we left the resin filled hearts to cure, the tissues could dry out and affect the strength and shape of the vasculature cast. This required us to let the resin cure in a warm and moist environment. After this step was taken, the degradation of the tissue went much quicker. In order to degrade the tissues of the heart, thus leaving only the resin casting behind, we submerged the casted hearts into a 2000ml beaker containing 1700 ml of 50% NaOH (w/v). We then let the heart sit in the solution with gentle stirring until the tissues were dissolved and the heart casting remained. Due to dissolved tissue residue buildup, we would change the solution about every 10 days. It was discovered that the tissues would dissociate from the cast and dissolve much faster if every third solution change would substitute 3x Phosphate Buffered Solution (PBS). I hypothesize that the proteins in the ECM change their conformation and weaken in the presence of such a concentrated salt solution. Although the exact mechanism is not known, the PBS wash greatly reduces the hold of the tissues to the cast, and make the next NaOH wash even more effective in removing tissues from the cast.
In future castings, I would try a resin that demonstrated all the aforementioned properties as well as hydrophobicity and a cure time that could be accelerated either chemically or with UV light. I would also like to try mitigating gravity’s deforming effect on the organ as it is being cast by pumping in our resin while the organ is submerged in water. This would allow the resin to cure without the weight of the heart pushing resin out of the veins and capillaries. There are many variations of the casting and degradation procedures that can be tried in order to yield a more detailed casting; however the casts we have made serve the purpose of proving that our decellularization technique doesn’t compromise the structure and integrity of the vasculature.
This is important because it is the vasculature that will be used to eventually reseed the ECM with the various types of human cells and cell media in order to create a viable organ. As this is an expensive and time consuming endeavor, the casting models we have created will also be used to map out appropriate sections of the heart for a smaller scale recellularization.
Figure 1 – The degradation process as observed on day 1, week 6, and final result