David Hallan and Dixon Woodbury, Department of Physiology & Developmental Biology
Introduction
Vesicle fusion is a key step in the cellular process of exocytosis and is at the center of neurotransmitter release by neurons. Fusion is driven by a set of proteins known as SNAREs which includes the protein SNAP25B.
In the synthesis and spectroscopic analysis of the SNAP25B protein, a strong, unexpected 260 nm peak has been seen. This 260 nm peak might correspond to DNA, RNA, or any other adenosine-containing molecule binding to SNAP25B. Previous work indicated that the 260 peak was not due to DNA or RNA. SNAP25B binding to an adenosine-containing substance could be the basis of an important regulatory process in exocytosis. Knowing whether or not ATP, ADP, or AMP binds to SNAP25B may provide imperative insight into the true mechanism of exocytotic release.
The purpose of this project was to (1) identify whether or not ATP, ADP, or AMP bind to the SNAP25B protein, (2) determine the rate constants of binding, and (3) understand how this binding may affect SNAP25B conformation and its relationship to the formation of the SNARE complex for exocytosis.
Methodology
To investigate the possibility of adenosine binding to SNAP25B, ATP was conjugated to the fluorophore trinitrophenol, or TNP. TNP is a fluorescent molecule that emits in the 530-560 nm range when excited at 408 nm. When TNP-ATP is in solution but not bound to a protein, this emission is very weak. However, once TNP-ATP binds to a protein, the TNP fluorescence is markedly enhanced. Thus, with enhanced fluorescence it can be seen whether or not a protein binds to ATP.
The bulk of our research plans made use of fluorescence spectroscopy using a fluorometer. Emission-acquisition scans in the TNP excitation/emission and tryptophan excitation/emission with a bandpass of 4 nm were first taken of a buffered solution (20 mM phosphate buffer at pH 6.4), then with 10 uM of TNP-ATP added to the solution, and finally with logarithmic additions of SNAP25B. Enhanced fluorescence intensity and a blue-shift in wavelength will indicate whether or not ATP is binding to SNAP25B, and changes in tryptophan fluorescence intensity or wavelength will indicate whether or not the protein is changing conformation as the single tryptophan in SNAP25B becomes more or less hidden or changes position. With increasing additions of SNAP25B, a saturation curve was observed, and from that saturation curve, using iterative functions, it is hoped kinetic equilibrium rate constants can be determined for the binding of SNAP25B to ATP. Competition studies were then performed to see if the TNP-ATP can be displaced by ATP, ADP, or AMP, and at what rates.
In addition to experiments using SNAP25B, other protein samples were run as positive and negative controls.
Results and Discussion
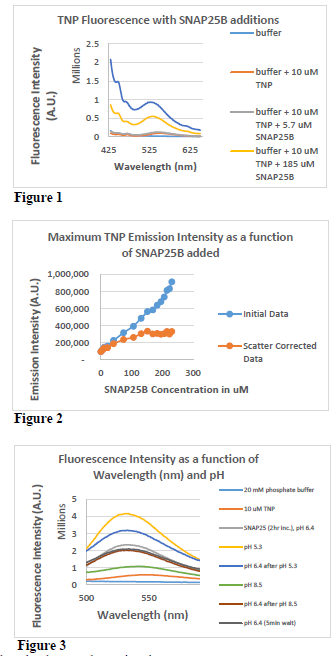
As seen in Figure 1, when SNAP25B was added to a solution containing TNP-ATP, the TNP fluorescence was greatly enhanced, and the maximal emission wavelength shifted from around 560 nm to 530 nm. Both of these are indicators that SNAP25B is an ATP binding protein. From this figure we also see that increasing amounts of protein cause a corresponding increase in light scattering, meaning that this variable will have to be corrected for in order to obtain accurate measurements.
Figure 2 shows that after correcting for light scattering the amount of SNAP25B that is required to saturate 10 uM TNP-ATP is about 150 uM. In other words, each mole of SNAP25B binds to about 0.067 moles of TNP-ATP. Unfortunately, when ATP was used to compete the TNP-ATP off in order to calculate rate constants, the light scattering the ATP caused was too severe to tell how much ATP was needed to do so (data not shown). This is a problem we are working on fixing.
Figure 3 shows that the effects of pH on SNAP25B / TNP-ATP binding. Binding is significantly increased at low pH (pH 5.3) and significantly reduced at pH 8.5. This effect can be recovered but takes time. Previous data has shown that pH changes cause SNAP25B to change conformation, which might explain a change in binding.
No changes in emission intensity or wavelength were seen during natural tryptophan scans.
Conclusion
SNAP25B binds ATP. Further research needs to be done to determine the rate constants of binding, whether ADP and AMP can bind, and the effects this binding might have on the SNARE complex and exocytosis. pH might also act as a regulatory mechanism
