Duval, Jared
Rational Design of Enzyme-like Bifunctional Peptide Catalysts
Faculty Mentor: David Michaelis, Department of Chemistry
Introduction
My research in the Michaelis Laboratory has focused on developing enzyme-like
multifunctional catalysts for organic synthesis. Nature makes catalysts (enzymes) capable of
chemistry that is not currently accessible by synthetic chemists. These enzymes take advantage
of preorganization, substrate activation, and proximity effects to enable fast and selective
transformations. Our goal is to mimic these biological processes in order to facilitate more
efficient and cost-effective reactions for the preparation of novel antibiotics and
chemotherapeutics.
Methodology
To enable enzyme-like catalysis, we use structurally well-defined helical peptides to scaffold
multiple catalysts (e.g. organo-transition metal, Lewis acid catalysts) in close proximity. Using
this design strategy, we combine the catalytic tools of organic synthesis with the exquisite spatial
control available to proteins to rationally design catalysts that facilitate faster and more selective
reactions and the development of new transformations. Our central hypothesis is that by
assembling multiple catalysts on a helical peptide scaffold, we can activate multiple substrates in
close proximity and enable the development of new coupling
reactions. The rationale behind our hypothesis is that helical
peptide templates enables precise tuning of inter-catalyst distances
and allows two catalytically generated intermediates to react
selectively with each other due to proximity effects. 1 To test our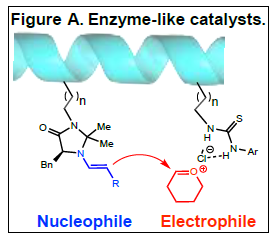
hypothesis, we have synthesized short (11-15 amino acid
residues) helical peptides and incorporated thiourea and
imidazolidinone catalysts capable of generating electrophiles and
nucleophiles in close proximity on the helix backbone (Figure A.)
My research has focused on developing methods to synthesize and purify bifunctional catalysts
like that seen in Figure A. Helix-forming polypeptides are synthesized on solid-phase using
microwave-assisted peptide synthesis. We then functionalize the peptide with organic catalysts
while still on solid phase using either azide alkyne click chemistry (imidazolidinone), or amine
functionalization (thiourea) strategies. Our small polypeptide catalysts are easily purified via
silica gel chromatography. We then ensured the peptide maintains its helical structure in
solution using circular dichroism.
Results
Our group has begun to confirm that our helical
peptide catalyst have unique catalytic activity. Our
initial studies have involved investigating the
reactivity of our bifunctional catalyst in the Diels-
Alder reaction shown in Figure B. In this reaction, the
imidazolidinone (Macmillan) catalyst activates an
aldehyde in close proximity with a diene that is bound
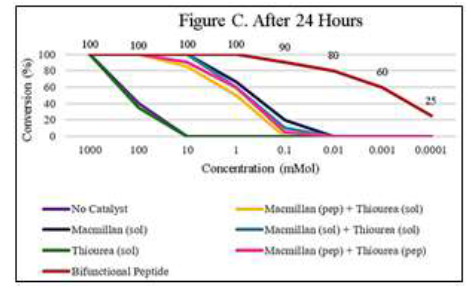
to the thiourea catalyst. In kinetic studies with this
catalyst system, we found that the bifunctional
catalyst maintains high reactivity at catalyst concentrations much lower than the when the two
catalysts are in solution (sol) or when they are on separate helical peptides (pep) (Figure C).
Discussion
My current work in the laboratory is to
investigate the impact of the helical peptide
structure on catalytic activity. I will
systematically vary the distance between each
catalyst by changing the length of the tether by
which they are bound to the helical peptide. I
will also investigate whether moving the
catalysts to the i+3, i+5, or i+6 positions on the
helix changes the catalytic activity of the
bifunctional peptide catalyst by moving the
catalysts farther apart. I am also currently
involved in the synthesis of new bifunctional peptide catalysts capable of conducting novel
bifunctional coupling reactions.
Conclusion
In conclusion, we have demonstrated that a rationally designed bifunctional helical peptide can
enable enzyme-like catalytic reactivity by pre-paying entropy costs through binding two reactive
substrates in close proximity. We have also demonstrated the critical nature of the helical peptide
structure in inducing these proximity effects, and have shown that our rationally designed
catalyst rivals the reactivity typical to efficient enzymes. Our proof-of-principle studies confirm
that helical peptides can serve as a platform for the rational design of bifunctional catalysts and
suggest the potential to incorporate any single-site catalyst (organo-, transition metal, hydrogen
bonding catalyst, Lewis acid catalyst) into this enzyme-like manifold. Continuing studies in our
laboratory are focusing on the design of new multicomponent reactions that rely on concomitant
formation of catalytically generated intermediates in close proximity on the helical peptide
scaffold.
1 Hilvert, D. “Design of Protein Catalysts.” Annu. Rev. Biochem. 2013, 82, 447–470.
