Siebach, Timothy
Visualizing Hemocyte Extracellular Traps in Galleria mellonella
Faculty Mentor: David Erickson, Microbiology and Molecular Biology
Introduction
Neutrophil extracellular traps (NETs) were recently found to be an additional method
that neutrophils use to combat pathogens. They are composed of nuclear DNA and
proteins that possess anti-microbial properties. Some studies suggest that in addition to
killing pathogens, NETs also serve as a barrier to prevent pathogens from spreading.
Mammalian infection models are known to possess NETs, but these models often raise
ethical issues and can be quite costly. Using an insect such as Galleria mellonella is a
valuable alternative. Insects possess an intricate innate immune response comparable
to mammals in many ways. There is already substantial evidence indicating that cells in
the hemolymph of G. mellonella phagocytose pathogens and produce antimicrobial
peptides. However, research regarding extracellular traps remains elusive. In this
project, we assessed the extracellular trap capabilities of G. mellonella larvae after
exposure to Escherichia coli. The results from this study will potentially provide further
support for using G. mellonella as an infection model.
Methodology
An incision is made in each larvae in order to collect the hemolymph in a centrifuge
tube. When at least 300μL of hemolymph is collected, a 1:10 dilution is performed into
Grace’s Medium (0.3mL of hemolymph and 2.7mL of Grace’s Medium). This solution is
gently mixed by pipetting up and down.
When the hemolymph is diluted, 500μL of the solution is added to a coverslip at the
bottom of each well in a 6-well plate. The hemolymph is left to sit for 30 min, allowing
the hemocytes to adhere to each coverslip. After this, various concentrations of
mammary pathogenic E. coli are added to four wells and left to sit for 3 hours. The two
remaining wells receive no E. coli.
After a 3-hour incubation, the liquid is removed from each well, and the coverslips are
gently washed with PBST (PBS with 2% tween). Next, 500μL of 4% paraformaldehyde
is added to each well and allowed to sit for 10 min on ice in order to fix the cells to the
glass. The paraformaldehyde is then removed and 500μL of chilled acetone is added in
order to permeabilize the cells while the 6-well plate is still on ice. After 3 minutes the
acetone is removed, and then 500μL of DAPI (15mL PBS + 5μL of DAPI) is added to
each well. The 6-well plate is placed in the dark. After 30 minutes, the DAPI is removed,
and the coverslips are placed onto slides for viewing under the microscope.
Results
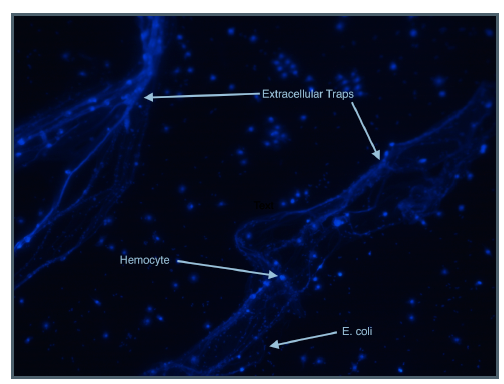
The top image was captured on a
fluorescence microscope after
hemoctyes were incubated with
mammary pathogenic E. coli for 3 hours.
The DAPI stain adds a blue
fluorescence emission to DNA. It
stained the DNA of both the hemocytes
and the E. coli as seen as the picture. It
also appears to have stained
extracellular DNA, suggesting the
presence of extracellular traps. We also
observed that the bacteria were more
concentrated around the DNA, which
indicates that it may indeed serve as a
barrier to prevent spread of bacteria.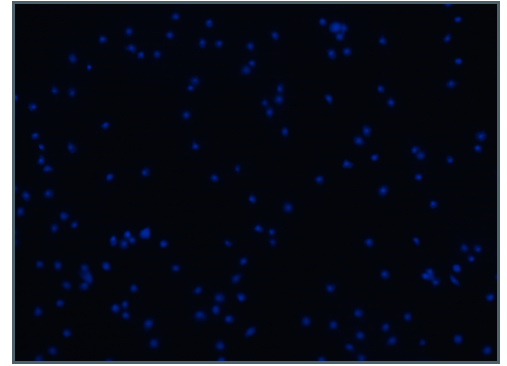
The bottom image was captured from a
sample of hemocytes that were not
incubated with E. coli. No extracellular
DNA was present on this slide.
In most instances, the hemocytes
incubated with E. coli exhibited
extracellular traps, and the negative
controls did not. However, there were
some occasions in which the opposite
occurred — hemocytes incubated with E.
coli did not display any signs of extracellular DNA or the negative controls showed signs
of extracellular DNA.
Discussion/Conclusion
The images captured under the fluorescence microscope present promising evidence
that Galleria mellonella larvae use extracellular traps as an antimicrobial defense
mechanism. Once consistent results are achieved, we would like to start treating the
samples with DNAse to see if the extracellular DNA is degraded. This will confirm that
DAPI is in fact staining DNA. We have also considered using anti-histone antibodies
with secondary fluorophore-containing antibodies to detect the presence of extracellular
histones that may be involved in the extracellular traps.
Although further evidence remains to be gathered regarding hemocyte extracellular
traps in Galleria mellonella, these larvae have already proven to be an inexpensive and
reliable alternative to mammalian infection models. In our lab, we have used the larvae
to discover virulence genes in mammary pathogenic E. coli.
Scholarly Sources
Altincicek, Boran, Sabine Stötzel, Malgorzata Wygrecka, Klaus Preissner, and Andreas Vilcinskas. “Hostderived
Extracellular Nucleic Acids Enhance Innate Immune Responses, Induce Coagulation, and
Prolong Survival upon Infection in Insects.” The Journal of Immunology (2008): 2705-712. Print.
Brinkmann, V. “Neutrophil Extracellular Traps Kill Bacteria.” Science (2004): 1532-535. Print.
Browne, Niall, Michelle Heelan, and Kevin Kavanagh. “An Analysis of the Structural and Functional
Similarities of Insect Hemocytes and Mammalian Phagocytes.” Virulence (2013): 597-603. Print.
Ng, Tze Hann, Miao-Hsien Wu, Sheng-Hsiung Chang, Takashi Aoki, and Han-Ching Wang. “The DNA
Fibers of Shrimp Hemocyte Extracellular Traps Are Essential for the Clearance of Escherichia Coli.”
Developmental & Comparative Immunology: 229-33. Print.
Ramarao, Nalini, Christina Nielsen-Leroux, and Didier Lereclus. “The Insect Galleria mellonella as a
Powerful Infection Model to Investigate Bacterial Pathogenesis.” Journal of Visualized Experiments JoVE
(2012). Print.