Paxman, Jason
Drunken Membranes: Short-Chain Alcohols Alter Fusion of Liposomes to Planar Lipid Bilayers
Faculty Mentor: Dixon Woodbury, Physiology and Developmental Biology
Introduction
The focus of my project was on understanding the effect of alcohol on the complex process of neural transmission, or how the body sends and receives signals through neurons. This essential communication occurs at junctions where neurons meet known as synapses. Essential to the process of sending any signal is the releasing of neurotransmitters into the synapse where they bind to receptors on the adjacent neuron. This releasing of neurotransmitter occurs through a process known as exocytosis. Exocytosis can only occur when neurotransmitter-containing vesicles fuse to the cell membrane. Alterations to the process of exocytosis can result in drastic consequences, such as the deadly paralysis seen in botulism. Initial experimentation with ethanol in our model system yielded dramatic consequences in inhibiting the fusion of vesicles and lead us to do more careful experimentation. The goal of this project was to carefully characterize this effect and submit it for publication in a scientific journal.
Methodology
To study ethanol’s effect on vesicle fusion I utilized an experimental procedure known as the nystatin-ergosterol fusion assay. This technique utilizes the cholesterol derivative ergosterol and the anti-fungal polyene channel, nystatin, to measure individual fusion events as vesicles fuse with the lipid membrane. Fusion leads to the insertion of the nystatin channels into the membrane. The flow of ions through these channels is picked up by electrodes on both sides of the membrane and the fusion event is registered as a current spike. We can then count the number of current spikes over time to calculate the rate of fusion before and after the addition of ethanol. This technique is appropriate as it enables us to quantify an experiments rate of vesicle fusion and thus run matched-pairs experiments to observe differences in fusion rate between control conditions and various concentrations of a given alcohol.
Results
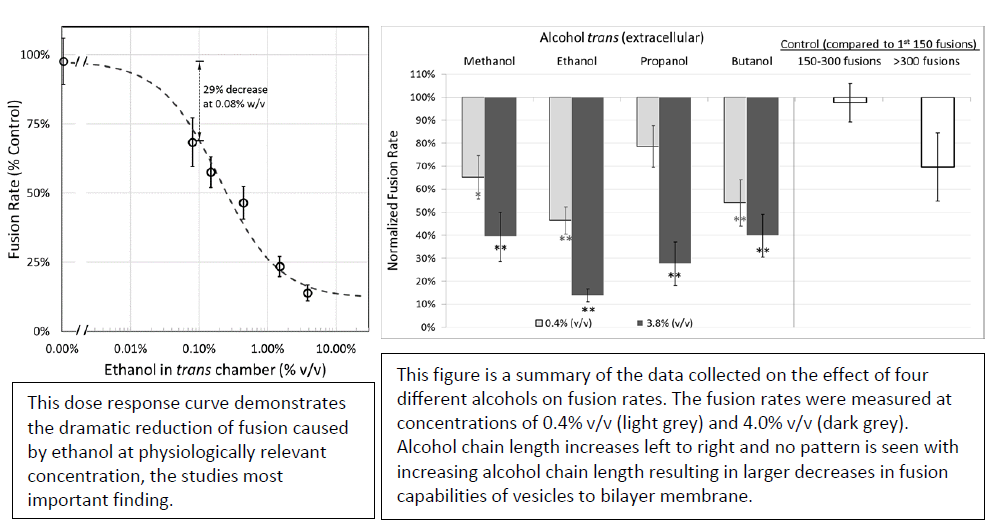
After many different fusion experiments involving many differing alcohols, I confirmed a few key parts of my hypothesis. First, the addition of alcohol on the intracellular side of our membrane greatly reduces the ability of vesicles to fuse. Second, the inhibition of alcohol was confirmed to not be due to an artificial osmotic effect, an important control in our experiments. Third, showed through process of elimination that alcohols are altering fusion by their direct action on membranes and not through some other means.
The one aspect of my hypothesis that I was unable to prove was whether increasing length of the alcohols added would increase their inhibitory effect on vesicle fusion. After extensive experimentation, the variability in our data was too large to draw any direct conclusions about potency as a direct function of carbon chain length, but all alcohols produced a significant reduction compared to controls.
Conclusion
In many past studies on the direct effect on lipid membranes caused by ethanol and other short-chain alcohols, relatively high concentrations of ethanol (500 – 2000 mM) were required to observe a measurable effect. The major finding of this research is that relatively low doses of ethanol (0.08% w/v or 17 mM) added to the membrane corresponding to the extracellular side are sufficient to cause a 29% inhibition in fusion rates of liposomes to planar lipid bilayers. Although these data do not prove that the pharmacological effects of ethanol on humans are due to a suppression of exocytosis and neurotransmitter release from neurons, the effect we observe in our simplified model of membrane-membrane fusion is large enough to provide a possible biophysical explanation of compromised neuronal behavior.