Angela Nakalembe and Dr. Alonzo Cook, Department of Chemical Engineering
Introduction
According to the latest U.S. Renal Data System Annual Data Report, more than 660,000 Americans are currently being treated for kidney failure, also called end stage renal disease (ESRD). The current treatment options, dialysis and kidney transplants, work well in some instances but have disadvantages, such as the inability to perform hormone regulation and potential organ rejection respectively.
The overall aim of our work is to create bioengineered kidneys that will provide alternative solutions to the current treatment methods without any of the drawbacks. New breakthroughs in the biomedical world support the theory that organs can be grown from cultured induced pluripotent cells (iPSCs). This is what we plan to accomplish as a research team. Our end goal is to be able to grow these kidneys from human patient cells such that they can have functioning kidneys for transplant without the risk of organ rejection. Specifically for this project, we aimed to successfully differentiate pluripotent stem cells into kidney cells that could be used to develop a functioning kidney.
Methodology
The first step was to obtain the iPSCs which were then plated on vitronectin, a glycoprotein found abundantly throughout the body. This helped provide an adhesive surface onto which the cells could latch in order to grow. The cells were cultured in a renal epithelial growth medium of different concentrations for a few weeks at a time (see Fig.1 for the set up). In addition, the cells in growth media were stored in an incubator to provide optimum conditions for differentiation. The medium used was comprised of Essential-8 (or E8, a solution containing the necessary nutrients for cell culture) and Renal Cortical Tubular Epithelial (RCTE) media that was made in the lab to aid in cell differentiation. We ran experiments with various ratios of the two solutions to see which would give the best results with regards to differentiation while still being economically viable. After various trials, we ultimately settled on a 50/50 ratio.
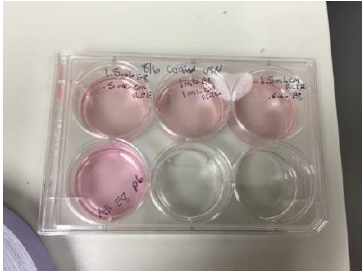
Fig 1: A 6 well containing iPSCs plated on vitronectin, with 4 different concentrations of growth medium. The well with 1 ml E8 and 1 ml RCTE media gave the best differentiation results.
Results and Discussion
Initial histology results for the cells in the 50/50 solution without any staining showed potential differentiation into kidney cells. To validate these results we ran the differentiation experiment again, this time staining the cells for Aquaporin-1(AQP-1). APQ-1 is a protein expressed in the human kidney in the endothelium and in the epithelium of the proximal tubule, and presence of this protein in our cells would confirm differentiation of the iPSCs to kidney cells. The histology results this time indicated the presence of fluorescent cells.
The presence of fluorescent cells under the microscope indicated the presence of AQP-1, which is exciting news for us as it shows that differentiation into kidney cells took place. However, the staining test alone is not completely conclusive, and to be absolutely sure of our results, a more accurate testing method than our lab can perform is needed. Therefore, our team has enlisted the help of Dr. Paul Reynolds’ lab in the BYU Life Sciences department to run a Western Blot test on our samples. Western Blot tests are high accuracy tests that use electrophoresis to confirm the presence of certain antibodies or proteins, and in our case AQP-1.
Conclusion
Preliminary tests for Aquaporin 1 confirmed stem cell differentiation into the kidney phenotype, which tells us we are on the right path with our research. A positive result of the Western Blot test will give us the green light to proceed with the next step, which would be to find ways to form kidney organoids from these cells.
I would like to thank Nafiseh Poornejad, Steven Passey, Matthew Nielsen, Kennedy Wandelt and Dr. Paul Reynolds for their guidance and assistance throughout this project. I would also like to thank Linda Palmer for the ORCA grant, and Dr. Cook for mentoring me throughout this project.
SOURCES
1. https://www.kidney.org/news/newsroom/factsheets/End-Stage-Renal-Disease-in-the-US
2. Clive.N.Svendsen. How human induced pluripotent stem cells will transform regenerative medicine. Oxford Journals 2013.
3. Nielsen.S. Kwon TH.Physiology and pathophysiology of renal aquaporins. Regenerative Medicine 2000; 2:289-300.
