Arthur Castleton and Alonzo Cook, Department of Chemical Engineering
Introduction
According to the American Heart Association, Ischemic heart disease is the principal cause of death. Many potential therapies are being simultaneously explored and among the most promising is tissue engineering of cardiac scaffolds. In vitro culturing of mature cardiomyocytes has the potential to lay the foundation for products such as cardiac patches for treatment of early stages of Ischemic heart disease or atrial septal defects, and developing entire replacement hearts. However, cardiomyocyte cell culture and experimentation has encountered various difficulties; the inability to proliferate, incomplete maturation, and having a small window of functional contraction. Conversely, iPSCs can partially differentiate into cardiac progenitor cells, adhere to a surface, then continue to proliferate prior to maturation into cardiomyocytes. Vitronectin is the most prevalent cell adhesive protein used for stem cell culture, however it is not usable as an implant scaffold or for clinical applications. Extracellular matrix (ECM) is a natural protein structure that provides a scaffold, growth factors, and other necessary components for tissues and organs. Due to the abundance of signaling proteins contained in ECM, it is a prime candidate to be used as a structural foundation for various cell lines. The objective of this project was to differentiate iPSCs into cardiomyocytes on Vitronectin or cardiac ECM (cECM) and then characterize the results to determine their relative feasibility as tools in future translational research.
Methodology
Cardiac ECM was decellularized in an automated perfusion bioreactor utilizing PBS, Type 1 water, Sodium Dodecyl Sulfate (SDS), and Triton X-100. IPSCs were partially differentiated into cardiac progenitor cells by culturing for 4 days in 6 well plates at 106 cells/cm2 at 37 °C and 5% CO2 in air; cell culture medium was replaced daily. A non-cytotoxic cell membrane dye was added to the medium to visualize cells infiltrating the scaffold. A million cardiomyocytes were then seeded onto 1 cm diameter by 200 μm sections of cECM (Figure 1) or Vitronectin in a 24 well plate, n=3 for each, then cultured for up to 2 months.

Figure 1. The method of differentiating iPSCs into cardiomyocytes and seeding them on the cECM sections (left) and a representative image of a segment of cECM on a 1 cm diameter coverslip (right).
Results
IPSCs differentiated into cardiomyocytes and seeded onto Vitronectin in a 24 well plate displayed mild contracture for only 15 days. Conversely, IPSCs differentiated into cardiomyocytes and seeded onto cECM contracted for the duration of the 2 month study (Figure 2). Cell viability assays showed that 75% of the 106 seeded cells adhered to the cECM, significantly higher than perfusion seeding methods reported in past studies. The cell viability had no definitive increasing or decreasing trends, indicating that the cells were likely stable and supporting the established knowledge that differentiated cardiomyocytes do not actively proliferate.
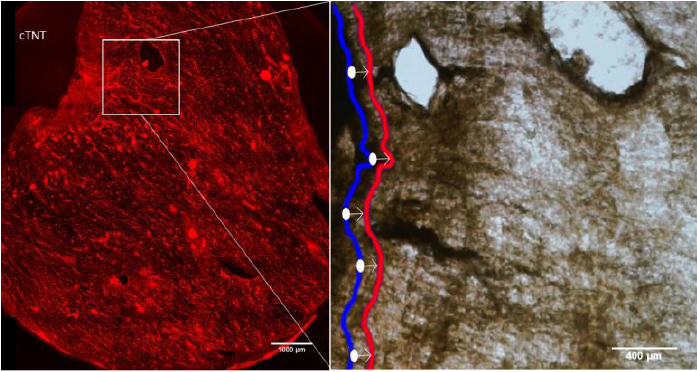
Figure 2. The left image shows the presence of differentiated cardiomyocytes throughout the cECM, represented by bright red. The right image shows the location of a segment of tissue when relaxed (blue) and where it moves to at its point of maximum contraction (red).
Discussion
A primary objective of cardiac regenerative medicine is to create transplantable tissue with a patient’s cells to eliminate potential for immunogenicity and to repair cardiac damages or abnormalities. To effectively accomplish this, scaffolds must be repopulated with all of the native cell types in the heart, not just cardiomyocytes. Appropriate placement of various cell types into their native positions is paramount in developing functional tissues capable of transplantation. Culturing human iPSCs on the cECM may be a practical method to produce a confluent cardiac tissue. It is expected that iPSCs cultured on cECM produce a higher quality constructive remodeling response than if seeded onto Vitronectin or other individual proteins because the cECM contains growth factors and other proteins specific to the heart that may help improve the duration and amplitude of contracture.
Conclusion
This study has demonstrated that slices of cECM from whole porcine hearts that were decellularized in an automated, pressure-controlled apparatus and cardiomyocytes derived from IPSCs seeded onto the cECM resulted in the cardiomyocytes’ contracture lasting much longer (60 days) than when seeded onto the academic and industrial gold standard, Vitronectin (15 days). This study may enable forthcoming researchers to study cardiomyocytes in a setting more similar to their native environment and enable the cells to further mature over longer periods of time. Future work will focus on comparing the ability of cardiomyocytes to adhere to cECM relative to other ECM scaffolds (e.g. porcine bladder) and utilizing electrical and mechanical stimulation to potentially enhance maturation of the cells.
