Lia Gale and Brian Jensen, Department of Mechanical Engineering
Introduction
Chronic wounds, particularly in the lower limb, represent a huge physical, financial, and social burden to 50 million people worldwide. In 2014, Americans paid an estimated $25 billion for simple wound care for patients. Despite these efforts, traditional methods of trying to heal wounds from the outside via surgical debridement, anti-inflammatory medications, moisture correction, etc. often fail to close wounds (Demidova-Rice et al., 2012). If wound closure does not occur, infection will cause localized tissue death and can lead to sepsis and death. Unfortunately, this leaves amputation as the only resort when wounds don’t close. If primary amputation occurs, the likelihood of a second amputation on the other limb is high, resulting in mortality rates as high as 69% five years after amputation. Thus,the key to solving this medical crisis is being able to get chronic wounds to heal in a timely manner.
This work seeks to approach the process of healing chronic wounds in a different way — healing wounds from the inside-out. This entails the implementation of two technologies: Lance Array Nanoinjection (LAN), a non-viral MEMS-based transfection technology; and CRISPR-Cas9, a nuclease editing system that allows for site specific modification of target cells’ genome. Specifically in this study, we plan to deliver a CRISPR-Cas9 plasmid that will transiently up-regulate production of a critical surface receptor (PDGF-β) on skin cells. This receptor is paramount to the healing cascade and is commonly degraded by native inflammatory processes found in chronic wounds. In order to deliver this plasmid, we will use LAN to insert the plasmid into primary neonatal fibroblasts.
Method
Nanoinjection Process
The general process of nanoinjection can be detailed in three major steps. First, using an array of 4 million, 10 micron long silicon etched lances, the lances are placed in solution with target cells and electrically charged to attract DNA to the lance. Second, the lances are then lowered into the cells, physically penetrating the cell membranes of the target cells. Third, now with the lance tips in the cell, the lances’ electrical charge is reversed causing release of the DNA into the cell. Following this, the lances are removed and the plasmid is allowed to operate.
Experimental Design
There are three major phases to this project. Phase I involves the acquisition of primary neonatal fibroblasts, the PDGFR-β CRISPR-Cas9 plasmid, and the fluorescent markers required for flow cytometry. Additionally, Phase I involves the culturing of the cells to obtain familiarity with growth. Phase II involves the nanoinjection of the cells. For this phase, there are two treatment groups and three control groups, which include:
- Treatment Groups: 3.0 or 4.5 mA current control, injected two times
- Control Groups: Non-Treatment Control, Background Control for Injection (LAN without DNA), Diffusion Control (LAN without electrical, +DNA)
Phase III involves taking the samples 48 hrs following Phase II to quantify and characterize via flow cytometry (Attune). Prior to flow cytometry, cells will be treated with fluorescent markers that help identify cell type (CD90) and levels of PDGFR-β. Data generated will then be analyzed in statistical software (JMP) to determine if there are any statistically significant relationships.
Results
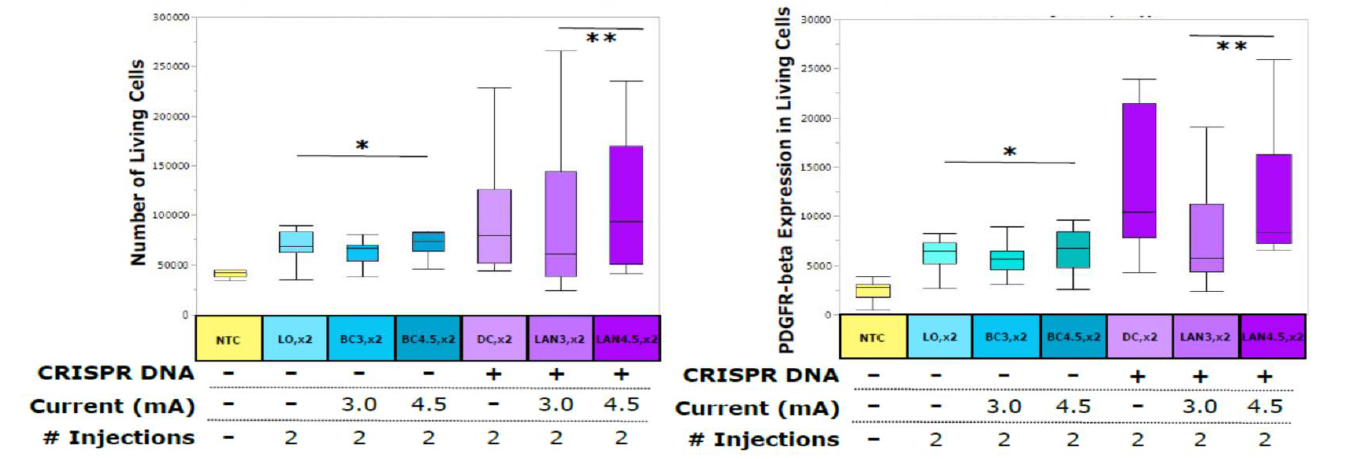
Figure 1. Flow Cytometry Analysis. The interpretation of the labels are as follows: NTC-Nontreated Control; LO,x2- 2 injections with no current or DNA; BC3,x2-Background Control, 2 injections with 3mA current; BC4,x2-Background Control, 2 injections with 4.5mA current; DC,x2-Diffusion Control, 2 injections with DNA present in solution; LAN3,x2- 2 injections with 3mA current and DNA present; LAN4.5,x2- 2 injections with 4.5mA current and DNA present.
Discussion
Essentially the results show that the LAN CRISPR-Cas9 system achieved PDGFR-β expression levels as high as 15.3-fold over the nontreated control (NTC). The number of living cells after cell proliferation is also as much as 3.18- fold greater in the sample type LAN4.5,x2 than the NTC.
Conclusion
This study shows that LAN is a highly efficient technique for introducing the CRISPR-cas9 system into the nucleus of a cell and upregulation of genes is possible using this technology. Transcriptional activation via CRISPR-Cas9 is new and has not been widely applied, particularly in the case of wounds (Konermann et al., 2015). Furthermore, no current technology can safely deliver CRISPR-Cas9 systems at the benchmark rates of adenoviruses, which cause immunologic responses. The one exception to this is LAN. Recent work shows that LAN can deliver a gene knock-out CRISPR-Cas9 plasmid to cancer cells, with expression rates of 93% (Sessions et al., 2015), a rate three times better than what has been reported with adenoviruses in the same conditions. This work confirms the effectiveness of LAN technology and its upregulation ability. This work also takes the next step in genetic engineering in this field by combining the advantages of LAN with the elegance of CRISPR-Cas9 into primary fibroblasts as a model for how to enhance chronic wound healing, and potentially to treat a variety of other skin conditions.
References
Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Advances in skin & wound care 25:304-14.
Konermann S, Brigham MD, Trevino AE, et al. (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517:583-8.
Sessions JW, Skousen CS, Price KD, et al. CRISPR-Cas9 directed knock-out of constitutively expressed GFP in HeLa cells using Lance Array Nanoinjection. Submitted to: PLOS ONE, October 2015.
