Jaron Lundwall and Alonzo Cook, Department of Chemical Engineering
Introduction
Due to accident-related neural damage, many people’s lives are impaired or limited in what they can do. Current medical practices are limited at helping distal and proximal nerve stubs regenerate. Many recent research studies have focused on trying to improve this problem by understanding how cut or crushed nerves heal. This study focused on helping these efforts by improving non-invasive analysis techniques of nerve growth. Magnetic Resonance Imaging (MRI) is one possible solution to creating a reliable analysis technique that in the future could be used on humans. We have shown that Magnetic Resonance Imaging can be used without invasive photoindicator injections to image nerve regeneration.
Current methods used to analyze the effectiveness of nerve regeneration techniques are often destructive to the animal being tested. The more invasive a technique, the longer it takes for the animal to heal. Some common techniques (e.g. histology) even require the death of the animal because the nerve is taken out in order to accurately measure how much their nerves have grown.
Studies have been performed using MRIs to study nerve regeneration. A study led by Dr. Matsuda showed that Manganese can be used as a biomarker as an injection into nerves to accurately measure how much nerves have grown [1]. This study however depends very heavily on the use of manganese to indicate on the MR image where the nerve is. Manganese can be a very inconvenient way to find nerves in tissue as most commonly it requires surgery to find the nerve and perform a direct injection into the nerve of manganese chloride. While the manganese itself is not toxic, surgery can be very taxing on the body and involves increased levels of risk to be able to monitor the growth of nerves. If MRI methods were to be applied more liberally to humans to measure nerve regeneration it will be needful that we find an alternative way to measure nerve regeneration without the need to perform surgery every time we want to take an image of the nerve. Along with this, the use of MRI to measure nerve regeneration could be invaluable to researchers everywhere that have access to MRI machines as an alternative to current methods which can cause inaccuracies and can delay accurate information until the end of the rat’s life.
Methodology
To analyze the ability of MRI machines to measure nerve growth we created three study groups that each undergo a different surgery to affect the sciatic nerve. The first group was of 12 rats and had their sciatic nerve completely severed (due to the elasticity of the nerve, a gap is formed between the two nerve ends). The second will be of 12 rats and again have a severed sciatic nerve and a silicon tube will be placed around both free ends of the cut nerve (this portion of the study is incomplete). The third group will be of 3 rats and underwent crushes of the nerve in order to act as control to match our current data and will undergo the MR scans at each time point until the end when they will be euthanized. Each of the different study groups had or will have their surgery type happen at 1.5-2 cm distally from the spine. The 24 rats of the first two groups were divided into subgroups of three in the time groups of 3 days, 10 days, 20 days, and 6 weeks with half of the 24 rats part of the first study group (severed nerve; no silicon tube) and the other half will be part of the second study group (silicon tubule). Thus making it so that at each time group there will be 3 rats of each of the two study groups. After the section of the nerve is severed according to each study group, the rat will then be sutured shut. Following the surgery, all 27 of the rats began to receive MR scans at each of the time points (3 days, 10 days, 20 days and 6 weeks). However, at each of the time points 6 of the rats (3 from study group 1 and 3 from study group 2) were removed from the study following their MRI and were euthanized and underwent histology to correlate our MRI data with the histology data. Thus the amount of rats decreased throughout the study with the remaining 9 rats (3 from the group 1-6th week team, 3 from the group 2-6th week team, and 3 from the control group) being euthanized at week 6. Four analysis techniques were used to control for the effectiveness of my MRI analysis technique. The four techniques were: muscle contraction reflex, Sciatic Functional Index (SFI), Magnetic Resonance Imaging (MRI) and histology.
When histology testing is taking place, we fixate the nerves overnight using glutaraldehyde followed by a two hour osmium tetroxide wash. The nerves then are dehydrated using acetone and then placed in resin. We slice the nerves using a microtone and then dye the nerves. Once the nerves are dyed, we analyze them using a light microscope and an electron microscope.
Muscle contraction reflex was tested by a simple prick in the leg, measuring the reaction time and how well the rat tucks their leg under themselves. Dr. Cook has a machine in his lab that we used that collected this data.
Sciatic Functional Index is a common, accurate test that was used to measure the healing of the sciatic nerve in our experiment. It is a simple test where the foot of the rat is placed in ink and then the rat is set on paper to walk on it and leave ink footprints. We then measure the spread of the toes from the footprints to measure the ability of the rat to use his foot normally.
The collected data from the mechanical and stimulus tests and the microscopy images will be compared with the images obtained from the MRI technique. A correlation will be drawn to relate the images to nerve functionality. This will allow for a standard to be created using just the details from the MRI images to assess nerve regeneration.
Results and Discussion
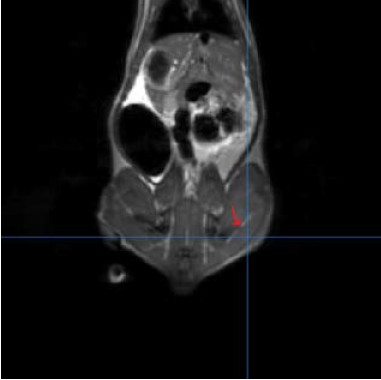
We were able to develop a method that does successfully and consistently image the severed nerves of the rats that we had in our study. The distances are measurable and average around 2 mm for each severed rat as shown in the picture with nerve regeneration distance still being measured (this is taken from the rats without the silicon tubule; the rats with the silicon tubule are still going through the study). We are currently compiling the data that correlates nerve regeneration that is seen in the MRI with nerve regeneration as detected by a muscle reflex test and a toe spread test (to measure functionality of the nerve).
Conclusion
We conclude, therefore, that it is possible to take MRI to image nerve regeneration without the use of resolving fluids. We anticipate that we will be able to correlate the regeneration data obtained in the MRI machine with our two other functional tests of the nerve so as to develop a method to be able to do rate functionality of a nerve based on an MRI image. A further study that we plan to conduct would be to wrap the nerves in a tube so as to promote regeneration of the nerve to further test our method.
1. Matsuda K, Wang HX, Suo C, et al. Retrograde axonal tracing using manganese enhanced magnetic resonance imaging. NeuroImage. 2010;50(2):366-374.