Kaitlyn Brower and Faculty Mentor: Adam Woolley, Department of Chemistry and Biochemistry
Preterm birth is the leading cause of death in children under the age of 51 and is defined by the Centers for Disease Control as birth before 37 weeks gestation.2 It is estimated that 15 million babies are born premature annually and that PTB kills nearly 1 million babies every year. Currently, accurate detection of PTB risk isn’t possible and extrauterine treatments and interventions are insufficient.
In 2011, researchers identified a panel of 9 preterm birth biomarkers that predicted preterm birth (PTB) risk with 86% sensitivity and 80% specificity.3, 4 One of these biomarkers is the iron binding protein ferritin. Ferritin is a hollow spherical protein made up of 24 subunits. There are 2 types of ferritin subunits, H and L. H subunits bind iron (Fe2+) and oxidize it to Fe3+. L subunits bind Fe3+ and initiate iron crystal growth inside the ferritin molecule. Studies suggest that higher H:L ratios occur as part of the body’s inflammatory response and higher L:H ratios occur in response to oxidative stress. 5 Currently, the biochemistry that links ferritin to preterm birth is not understood. Identifying the H:L subunit ratio in PTB cases may help elucidate the connection between ferritin and PTB.
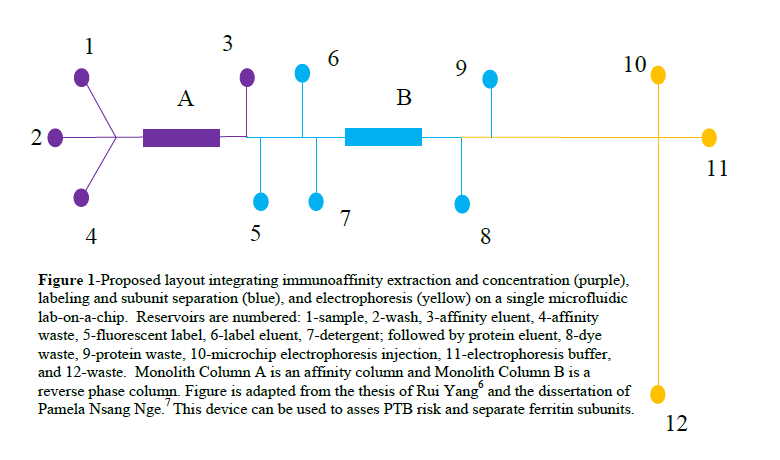
We are working to develop a device that can be used to investigate the relationship between ferritin and PTB by assessing the H:L subunit ratio and assess PTB risk in a clinical setting. Figure 1 depicts the proposed layout of the device. The first module will be used to extract the biomarkers from serum and concentrate them using solid phase extraction. The second module will use a reverse phase monolith to retain the biomarkers so they can be fluorescently labelled easily. Finally, the third module will be used to separate the biomarkers. When quantifying ferritin subunits, an additional step will be added between columns A and B in which detergent will be used to separate the subunits before labeling and separating them. The scope of this study was optimizing retention, elution and detection using the reverse phase monolith in the second module of the lab on a chip device.
The device fabrication processes have been developed in the ATW lab.6, 7 The first step in fabricating these microfluidic devices is template fabrication. First, a positive photoresist is spin coated onto an oxidized silicon wafer. Next, a mask is applied and the photoresist is exposed to UV light and then developed with S1805 developer. This leaves photoresist protecting the oxide layer where we want the channels to form. Next, the excess and unprotected oxide layer is removed from the wafer with HF etching. The protective photoresist layer is then rinsed away with acetone and KOH etching is used to etch the channels to the desired height.
Hot embossing is then used to imprint the channel design into cyclic olefin copolymer (COC). This is done at 138 °C for 24 minutes. Reservoirs are drilled into a second COC plate and this plate is flattened at 138 °C for 24 minutes. Once both plates have cooled, they are thermally bonded together for 28 minutes at 110 °C to form the channels.
The next step was developed by fellow group members Dr. Radim Knob and Mukul Sonkers. After the channels have been formed, they are filled with a solution that has 1% benzoin methyl ether, 5% polyethylene (glycol) diacrylate and 94% methanol and subjected to UV light for 8 minutes. The excess solution is removed with vacuum and washed thoroughly with IPA. This process increases surface hydrophilicity and reduces protein adsorption, which inhibits protein separation.
After the columns have been coated, monolithic columns are synthesized. Columns are formed by adding 2,2-dimethoxy-2-phenylacetophenone in a 1% ratio to a solution that is 20% octyl Methacrylate, 10% ethylene dimethacrylate, 25% cyclohexanol 20% Tween 20 and 25% 1-dodecanol. The column is filled, a mask applied and the device subjected to UV light for 12 minutes. The excess solution is then removed with vacuum and the column washed repeatedly with isopropyl alcohol.
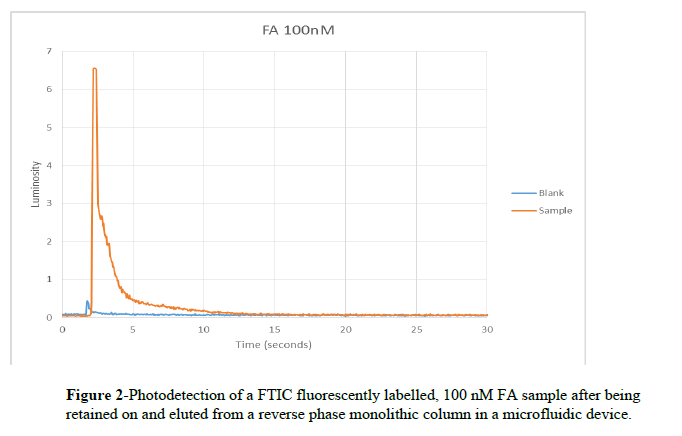
FA, a peptide, was labelled with Fluorescein isothiocyanate and diluted to 100 nM. After the column was washed and equilibrated, a buffer wash was captured to be used as a control with 50 mM carbonate buffer pH 10. The column was then equilibrated with 50 mM citrate buffer pH 5 and the pre-labelled sample was loaded onto the column using 400 Volts and allowed to incubate for 3 minutes. The column was rinsed with citrate buffer and then the sample was eluted with carbonate buffer at 400 V. Elution of the sample was detected using a laser, microscope, and photomultiplier tube detection system and data was recorded using Labview software and analyzed with Excel.
Figure 2 shows successful retention, elution and detection of FA at 100 nM. As studies have shown that preconcentration of factors of 103 are attainable by using solid phase extraction in microfluidic devices8 (column A in Figure 1), detection of 100 nM samples demonstrates that retention, elution and detection at physiological levels of ferritin (0.6- 175 ng/mL for women)9 is feasible using this procedure.
Further studies will include testing a butyl methacrylate column for retention, elution and detection of FA to see if tailing can be reduced and detection improved. Based on those results, the columns will be adapted to optimize the retention, elution and detection of ferritin on both octyl methacrylate and butyl methacrylate columns. Once optimized, this module will be integrated with the other modules of the lab-on-a-chip device.
Works Cited
(1) Preterm birth. World Health Organization, http://www.who.int/mediacentre/factsheets/fs363/en/ (accessed Dec 20, 2015).
(2) Preterm Birth. Centers for Disease Control and Prevention, http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm (accessed Dec 20, 2015).
(3) Esplin, M. S.; Merrell, K.; Goldenberg, R. L.; Lai, Y.; Iams, J. D.; Mercer, B.; Spong, C. Y.; Miodovnik, M.; Simhan, S. N.; Van Dorsten, J. P.; Dombrowski, M. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2011, 204, 391.e1-8.
(4) Graves, S. W.; Esplin, M. S. Validation of predictive preterm birth biomarkers obtained by maternal serum proteomics. Am J Obstet Gynecol. 2011, 204, S46.
(5) Watt, R. K. The Many Faces of the Octahedral Ferritin Protein. Biometals BioMetals. 2011, 24, 489–500.
(6) Yu, C.; Svec, F.; Fréchet, J. M. J. Result Filters. National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/pubmed/11721904 (accessed Dec 20, 2015).
(7) Ferritin blood test: MedlinePlus Medical Encyclopedia. U.S National Library of Medicine, https://www.nlm.nih.gov/medlineplus/ency/article/003490.htm (accessed Dec 20, 2015).
(8) Nge, P. PhD. Dissertation, Brigham Young University, 2012.
(9) Yang, R. Masters Thesis, Brigham Young University, 2014.