Brady Vance and Alonzo Cook, Chemical Engineering
Heart disease, according to the Centers for Disease Control and Prevention, is the number one cause of death in the United States, killing more than 600,000 people each year1. Due to the high incidence of heart disease and the low availability of transplantable hearts, new technologies have been developed to lower the mortality rate of the disease. Even though there have been significant improvements to surgical procedures and treatments for heart disease in recent years the need for more effective solutions is evident. One such method is heart tissue engineering.
Our research team is using porcine hearts to study the processes of decellularization and recellularization of native scaffolds with an end goal of producing a new method for organ transplants. Using the decellularized Extracellular Matrix (ECM) as a scaffold, our objective is to repopulate the scaffold with a patient’s cells. This is a complicated process due to the fact that there are several cell types present in a native heart. In order to accomplish this goal, steps must be taken to ensure that each cell type will be able to grow and proliferate on the decellularized scaffold. Fibroblast cells are one of the main cell types found in the heart and their prime function is to increase collagen concentrations in a native heart. Collagen is a type of connective tissue and acts as a thin covering to cardiomyocytes, which helps to create ideal conditions for heart contractions.
For my ORCA project I recellularized small sections of porcine right ventricle with human cardiac fibroblast (HCF) cells. First, using a cryostat, I cut frozen pieces of right ventricle to a thickness of about one millimeter. These thin slices were then made into small circles using a biopsy punch. After placing the sample on a glass coverslip to secure the ECM into place, the samples were put into a 48-well plate. In order to create a sterile and safe environment for cellular growth, the pieces of ECM were then sterilized with ethanol. The ethanol was rinsed away using DMEM and cell media.
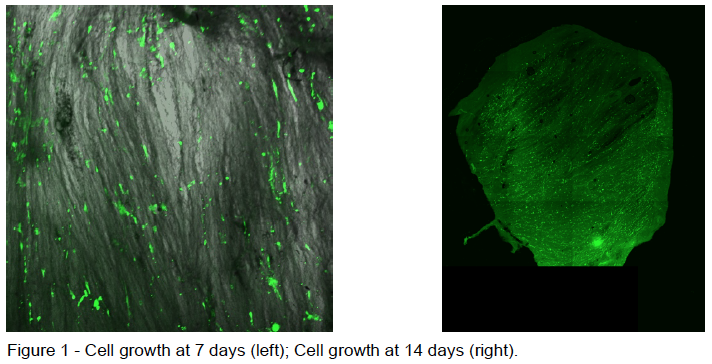
Prior to recellularization, I cultured HCF cells in a flask. Once there was a sufficient number of cells, I placed them onto the prepared ECM samples. Cell media, which allowed the cells to stay alive and continue proliferating, was changed every 2 days. After 4 days of growth I began to take 4 samples of the recellularized ECM every 2 days for 20 days. Samples were taken by removing the ECM from the 48-well plate and freezing them in a microcentrifuge tube. I obtained 10 samples total over the 20-day period, including a negative control which had no cells on the ECM. I also took 2 additional samples of which I took images in order to insure there was cell growth throughout the experiment (shown below in Figure 1). Once all of the ECM samples were gathered they were freeze dried in a lyophilizer. After the samples where in the lyophilizer for 2 days, I removed them and ran a hydroxyproline assay in order to detect the amount of collagen growth on each of the samples. Hydroxyproline is a very common amino acid found in collagen and therefore it is a good measure of the amount of collagen present in a sample. The absorbance of each well was taken at 560 nm in a well-plate reader.
My hypothesis was that ECM collagen concentration would increase in a linear fashion as growth time increased for the HCF cells. After conducting my project, I learned that my hypothesis was incorrect. There was a slight positive correlation indicating that overall collagen increased, but the result was not statistically significant. After comparing the data to the standard curve that was prepared beforehand, the amount of collagen present in the samples was between 0.83 and 0.97 μg/well. Although this was different than the anticipated result there was an increase of collagen as compared to the negative control which had .79 μg/well. This means that there was collagen production during the experiment.
Looking forward, I would like to determine the amount of collagen that is preserved during the decellularization process. This could be accomplished by quantifying the average amount of collagen present in a native heart and how much on average is present after decellularization. If we are able to preserve a significant amount of collagen after decellularization, it could explain why we didn’t observe a more linear increase in collagen concentration during the previous experience. It is possible that our ECM samples were already saturated with collagen before the experiment began.
Collagen plays an essential role in the function and structure of the heart. With regard to the recellularization process, it is important that we can replicate the conditions found in a native heart so that our porcine heart alternative will be fit for human transplantation. I believe this experiment represents an important part of our goal to recellularize a porcine heart with human cells. This experiment has also given us a direction to proceed in order to determine whether or not the ECM will contain sufficient collagen to support the recellularization of a full heart.
- 1 Centers for Disease Control and Prevention. Leading Causes of Death. Available at http://www.cdc.gov/nchs/fastats/leadingcauses- of-death.htm. Accessed October 23, 2014.