Dallin Lindstrom and Brian Jensen, Mechanical Engineering
Introduction
A silicon nanoinjection lance array has previously been developed to deliver charged propidium iodide (PI) molecules into tens of thousands of HeLa culture cells simultaneously. DNA is also charged, so applying a voltage to the lance array attracts DNA molecules to the lances. The voltage can be reversed to repel DNA molecules away from lances and into cells. Since carbon nanotubes have a more porous surface than silicon, it is hypothesized that carbon nanotube lances will be able to hold more DNA molecules than silicon lances, and therefore be more efficient at getting the DNA into the cells. The purpose of this project is to determine if a carbon nanotube lance array has an effect on the efficiency of the nanoinjection process.
Methodology
To fabricate the lance array chips used in this project, micron-wide solid lances (solid needles) are etched into a silicon wafer using MEMS fabrication technology. One 2cm x 2cm silicon chip contains millions of lances. In contrast, carbon nanotube lances can be grown on a silicon substrate to result in similar lance dimensions but a porous lance body. The chip is then used to inject HeLa cells with the GFP DNA Plasmid. GFP is a plasmid that results in the production of a fluorescent protein which allows us to measure how many cells are expressing GFP after the injection process.
HeLa cells are cultured in six-well plates, where they adhere to the base of the well. Cells are then rinsed with saline solution; more saline solution is added to each well, as well as 2 micrograms/milliliter of GFP Plasmid. For one round of injections, two six-well plates are used. Two types of samples are needed for each test: negative controls (no GFP plasmid), and injected samples (GFP added). Positive controls are measured by the flow cytometer and only need to be set in the machine periodically. The controls give us baselines to measure against for GFP expression in injected samples. The chip is mounted to an injection device. Employing compliant springs and a stepper motor, the injection device allows the lances to be pushed into the culture of cells and released easily.
A constant current of +1.5 mA is applied to the silicon lances to attract the negatively charged GFP Plasmid for 20 seconds. The lances are then pushed through cell membranes and a short pulse of ten pulses between -1.5 V and -7 V are applied within 20 milliseconds. The short pulse gives a stronger repulsion force without the toxic effects of a higher voltage on cells. Following the pulse, a constant -1.5 V (resulting in 3 mA) is applied for 5 seconds, and finally the lances are removed from the cells. The only difference in protocol between the silicon chips and the CNT chips is that because the CNT chips have a greater conductivity, they require a lower voltage to achieve the same current. As such, the same current is used to attract, and the same pulse is used as in the silicon chips, but only -.8 V (also resulting in 3 mA) is used to repel the plasmid.
After the injection process, the cells are allowed to incubate for 24 hours to allow full GFP uptake and cell division to ensure the GFP has been incorporated into the cell’s genome. After 24 hours, Trypsin is added to each well to detach the cells from the bottom of the well. The contents of each well are added to separate FACS tubes and centrifuged to allow the cells to congregate at the bottom of each tube. The contents of each tube are run through a flow cytometer, which discriminates between GFP positive and GFP negative cells. Flow cytometry data is then analyzed to determine what percentage of cells expressed GFP.
Results
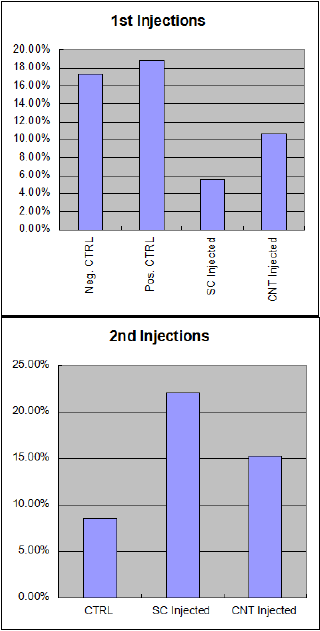
Results from the first two rounds of injections showed that there were higher control levels of GFP expression than there were in the injected samples. This was due to an error in the protocol that is discussed below. However, it also showed a greater efficiency in transfection in the CNT arrays over the silicon arrays. After the error in protocol was fixed, the control numbers went down to expected levels, and the injected transfection efficiency went up as well. At this point, the silicon arrays were performing better than the CNT arrays, which is also discussed below.
Discussion
Initial results showed that we needed a change in our methodology regarding controls. The first table to the right showing the first two injection sets shows that there were controls that exhibited higher GFP percentages than those that had been injected. This was obviously a false positive because the control wells never received GFP. Concurrent testing on a different project with the same protocols revealed that because, initially, our controls were not being injected, the cells were not being ripped off while the injected samples were, and were overgrowing the plate and producing false results. Because of this, we changed our control protocol to include injections in the control wells, but still not add any GFP plasmid.
After we changed that, the control numbers went considerably lower: from around 18% down to around 8%. The injected cells also began to show higher numbers, but unfortunately it was discovered that the CNT chips had lost the majority of their needles due to overuse, so their transfection efficiency were no longer larger than that of the silicon chips.
Conclusion
Further testing is needed combining the new, better protocol, as well as newly fabricated CNT chips. This will be able to definitively show which chip is better suited to GFP transfection in HeLa cells. Because we don’t have a technician to make CNT chips at this time, I was unable to proceed with these findings. Initial testing did uncover a large improvement that was needed in the protocol as well as promising transfection rates using CNT arrays, which are also more biologically suited for cellular interaction. We also now know that CNT chips can be used to a maximum of two rounds of injections before they need to be replaced.