Taylor Woodward and Scott Steffensen, Psychology
Introduction
Addiction is a diabolical neurochemical trap that robs people of their agency and catastrophically affects virtually every aspect of an addict’s life. Alcohol is one of the most statistically widespread and harmful addictive substances in our society (Nutt, King et al. 2010). Through physiological means, it destroys an addict’s dignity, disrupts the ability to maintain healthy relationships, and often results in premature death of the addict and those around him or her. M. Russell Ballard recently spoke about the spiritual implications of the pleasure center of the brain, stating that “when activated by certain drugs or behaviors, it overpowers the part of our brain that governs our willpower, judgment, logic, and morality. This leads the addict to abandon what he or she knows is right” (Ballard, 2010). We are in the midst of trying to understand how drugs of abuse affect the brain, and the findings of this project aided in furthering our understanding of the neurobiology of addiction. Thus, we are one step closer to finding pharmacological treatments to help addict reclaim their agency and their lives.
Studies have shown that drinking and smoking are correlated; drinkers are more likely to smoke, and vice versa (Bobo, 2000). My project cast light on one of the molecular mechanisms behind how EtOH affects the brain, which is not well understood, by referring to the well-studied mechanisms of NIC addiction. Specifically, we looked at the role of a6 subunit-containing nicotinic cholinergic receptors (α6*-nAChRs) in the ventral tegmental area (VTA), a brain region that is hijacked by drugs of abuse. Nicotine acts on these receptors, and we were able to show that ethanol (EtOH) at small doses was able to have an effect on them as well.
Methodology
To perform these experiments, we measured electrical signals called evoked inhibitory post synaptic currents (eIPSCs) in VTA GABA neurons in brain slices. We used mice (i.e., GAD GFP) that enabled us to visualize VTA GABA neurons under a microscope. Mice were exposed to chronic intermittent EtOH in our lab’s alcohol vapor chambers, which we used to administer EtOH and induce alcohol dependence. Their brains were extracted during peak withdrawal phase, and then sliced using a precise sapphire blade. The slices were placed in a solution of artificial cerebrospinal fluid at 36°C to mimic physiological conditions and keep them viable during testing. A method called patch clamping was used to record VTA neuron firing rate and evoked synaptic responses (e.g., eIPSCs), which involves inserting a micropipette with a recording electrode into a single neuron.
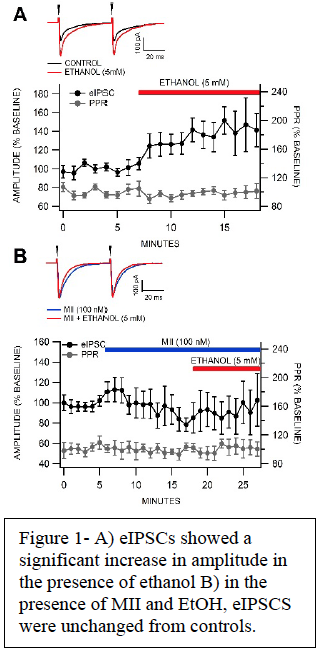
With our protocols we measured the eIPSCs or the amount of inhibition in a VTA GABA neuron. Specifically, we measured the change in IPSCs that occur in the presence of alcohol both when nAChRs are functioning and when they were nonfunctional due to the presence of potent and specific conotoxins, including MII, which blocks α6*-nAChRs. We evaluated measures of inhibitory GABA-mediated synaptic transmission to VTA GABA and DA neurons in alcohol-dependent mice vs air-exposed controls.
Results/Discussion
We expected that chronic alcohol would increase EtOH-induced eIPSCs and that in the presence of MII with alcohol eIPSCs would exhibit no change from controls. After experiments were completed and analyzed, we found our hypothesis to be correct. MII was effectively able to block the increase shown in the presence of alcohol. Because MII is a super-specific blocker of α6*-nAChRs, we can conclude that these receptors play a part in EtOH’s effects on the VTA.
Why is this important? This shows a common molecular pathway in in EtOH and nicotine reward systems, meaning that treatments might be effective in treating more than one addiction at a time. Further experiments could be performed to determine whether other drugs of abuse act upon similar pathways or what commonalities may be shared in order to find an effective pharmacological treatment for addiction.
I was able to present findings of this research at the Society for Neuroscience , an experience that changed my life and solidified my desires to pursue research as a career.