Eli Schriever and Scott Steffensen, Psychology
Introduction
One of the primary focuses of much addiction research involves finding methods to alleviate methamphetamine (METH) addiction. METH is one of the most widely used illicit drugs in the United States. Estimates by the National Institute on Drug Abuse Statistics are that 5.5% of all adults ages 26 and above have at tried METH at least one time. Given its high addictive potential, the chance for entering damaging addiction cycles among these individuals is very high, and can have great societal and economic consequences in addition to physical and mental problems on the individual level. While the need for better preventative education cannot go understated, the need for methods to help attenuate the addiction would provide an important tool to help addicts break METH’s vicious cycle. The rapid release of dopamine (DA) in neurons of the brain’s pleasure centers the Nucleus Accumbens (NAc) and Ventral Tegmental Area (VTA) after exposure to METH is widely documented, and has been implicated in the associated addictive behavior, but the mechanism of this action isn’t well understood. Our area of interest is based on the finding that after exposure to METH, reactive oxygen species (ROS), which includes hydrogen peroxide and free radicals, increases dramatically, bringing about oxidative stress, which may have a downstream effect leading to increased DA release. The body naturally uses glutathione peroxidase to reduce the damage caused by ROS, which oxidizes glutathione as a cofactor. Our hypothesis was that by adding Glutathione (GSH) to the system, we would be able to increase glutathione peroxidase activity and attenuate the effects of METH on DA release.
Methods
In order to examine GSH’s effects, we used ex vivo voltammetry to measure dopamine’s release in the NAc of wild type mice. DA is oxidized and reduced at specific voltages, so by passing a small current through an area of interest and measuring the voltage change, we can measure relative concentrations of dopamine for that area. The experiments had to be performed ex-vivo because glutathione doesn’t cross the blood brain barrier, meaning that performing the experiment on a live subject wouldn’t have allowed us to deliver the drug to our target area. In preparation, wild type mice which hadn’t been exposed to METH previously (simulating a first time user), were first anesthetized using isoflourane inhalation before having their brains removed and placed in a glucose rich artificial cerebrospinal fluid solution (ACSF). Brains were then cut into slices 400 μm slices using a vibrotome and then placed into a voltammetry chamber with a constant flow of ACSF over them to preserve the slices. In this way, substances such as GSH or METH can also be introduced to the slice by adding specific amounts to the ACSF being passed across the voltammetry chamber. Recording carbon fiber electrodes (CFE) were then inserted into the NAc along with a glass capillary stim to introduce current. Stimulating currents were applied at specific calibrated frequency and duration so as to be able to measure dopamine oxidation. For this experiment, GSH was first added at 100 μM concentrations and the voltage signal was allowed to stabilize before adding 5 μM METH. Both phasic and basal responses were recorded as part of data collection.
Results
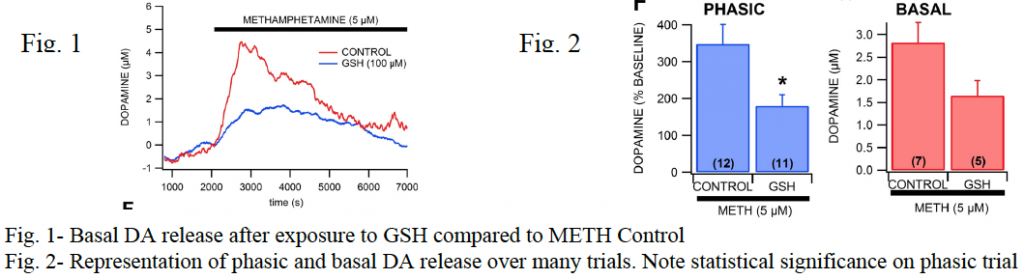
After analysis of data was completed for both phasic and basal release parameters, a significant difference was observed between DA release for control 5 μM METH exposures and samples in which GSH had been added first. Typical 5 μM METH exposure brought DA release to approximately 340% of baseline levels, but when slices were first exposed to glutathione, DA levels averaged only about 180% of baseline concentrations, which was statistically significant. In addition, basal response was measured, which involves a similar procedure as in the one listed above, except that instead of many short stimulation and data collection cycles, one collection was made over a several hour period. This process helps us record DA release enhancement over time as well as allows us to correlate voltage to actual DA concentrations. In the basal experiments, GSH reduced DA release from 2.8 μM to 1.6 μM compared to controls.
Discussion
These findings carry several important implications for addiction research. They show that antioxidants seem to be able to block some of the downstream effects of methamphetamine usage. In order to further investigate these effects, our laboratory has concurrently been investigating the use of TEMPOL, which is another antioxidant, though rather than acting as a substrate for an endogenous enzyme, TEMPOL is a stable radical species which scavenges other free radicals and thus is known to be able to reduce oxidative stress. We have observed TEMPOL having similar effects in reducing DA release after METH exposure. Because of the converging patterns of both of these two antioxidant species that use different mechanisms, it seems likely that ROSs do indeed play a role in DA release.
Conclusion
The fact that DA production is decreased as a result of GSH exposure doesn’t mean that GSH is a viable treatment option for METH addiction. As it doesn’t cross the blood brain barrier, its administration wouldn’t have any effect under normal biological conditions. However, these findings may give us a greater understanding of the mechanisms that cause addiction, and thus, bring us closer to future treatments. In the mean time, we will continue to use the data we have collected to draw other conclusions and find new areas for research as there is likely many factors that play a role in METH addiction. Through these efforts, as well as collaborating with other researchers involved in this field, we hope to be able to develop better treatment options for those struggling with substance abuse.