Krisana Finlay and Scott Steffensen, Psychology Department
Introduction
The purpose of this experiment was to determine the action mechanism of the psychostimulant methamphetamine (METH) on basal and phasic dopamine (DA) release in the nucleus accumbens. We investigated METH and its method of action. This was done to better understand the detailed mechanism behind METH and to provide better addiction-solving tools. Previous METH research provided a great starting point, showing a reduction in DA transmission and tyrosine hydroxylase, and increased DA axon and terminal damage, apoptosis (cell death) and axon degeneration.
Background
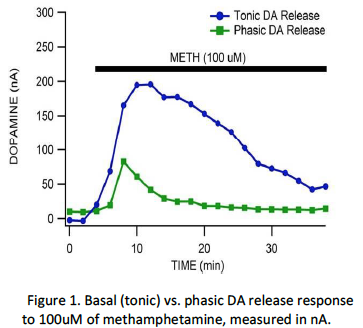
Acute administration of METH markedly enhances DA release in the nucleus accumbens of the striatum, the target of DA neurons originating in the midbrain ventral tegmental area (VTA). Dopamine releases in two patterns, basal and phasic. Basal dopamine release is a steady release state, a tonic level of extracellular DA generated by low-frequency neuronal impulses or spontaneous release. Phasic DA signaling through high-frequency bursts of stimulation (in this case artificial stimulation by an electrode) causing mass release of extracellular DA. Both basal and phasic release is concentrated in the synaptic cleft, the communication area between two neurons. In response to METH, DA phasic responses decrease while basal responses do not (Howard et al., 2011). We investigated these basal vs. phasic differences in reaction to acute METH via voltammetry. This was done using glutathione (GSH), tetrabenazine (TBZ) and GBR 12909 dihydrochloride (GBR). GSH is a required substrate for the glutathione peroxidase antioxidant system, while TBZ and GBR are VMAT and DAT blockers respectively. DAT, a transmembrane protein found in the presynaptic terminals, is known to regulate DA reuptake in the synaptic clefts. The dogma is that METH enhances DA release by actions on both VMAT and DAT. Specifically, METH blocks DA uptake into vesicles and reverses DA uptake into synaptic terminals. Ultimately, DA levels rise in the nerve terminals and flow out of the DAT. We developed a mechanism to analyze basal and phasic DA differences in response to methamphetamine, presented in preliminary data recently analyzed, shown in Figure 1.
We hypothesized GSH would decrease METH enhancement of DA release via its antioxidant properties. TBZ will decrease phasic DA release while GBR will decrease basal (or tonic) DA release.
Animals
Experimental protocol for this study required living brain tissue slices from wild type mice. The mice used in these experiments were C57/BL6 (wildtype) mice aged 18-22 days.
Experiment
Based upon preliminary data and aforementioned patterns of DA and METH effects, we tested the basal vs. tonic DA response to METH with the three compounds listed above. This was done by mouse brain extraction and slicing of brain tissue into 400 um. Slices were kept in a chamber with constant flow of artificial cerebral spinal fluid (ACSF). Kinetic analysis of DA responses was measured using fast-scan cyclic voltammetry (FSCV), stimulated with a lowfrequency electrode, with DA oxidation rates picked up with a carbon-fiber microelectrode (CFM).
Results
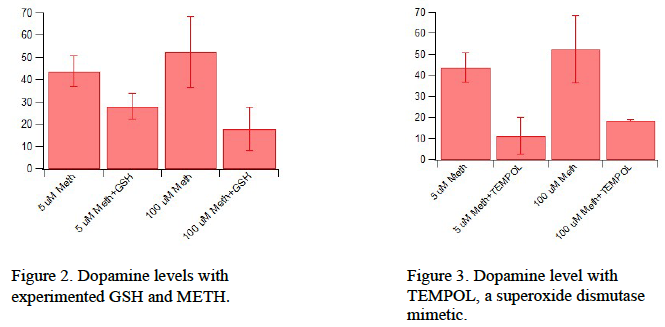
Both GSH and TBZ show no significant phasic effects (Figure 2 & Figure 3). Basal experiments were not yet conducted with GSH and TBZ, but follow-up experiments are currently under way.
Conclusion
We believe that METH is creating reactive oxidative species (ROS), although we are not sure how METH is creating ROS in the dopaminergic system (increased ROS limits a cells vitality and function). There are correlates that with increased METH comes increased ROS, which then, as we believe, pumps the gas for basal DA release. There are many types of ROS compounds in the brain. We believe the ROS interacting with METH and DA, is hydrogen peroxide. Through experiments, we found that hydrogen peroxide is decreased by GSH/GPx action. This supports the theory that GSH/GPx is a buffer against METH and other drugs actions. We hope to find additional experimental data that backs this theory.