Grant Ludlam and Faculty Mentor: Barry Willardson, Department of Chemistry/Biochemistry
Introduction
The protein Raptor is an essential component of the mechanistic target of rapamycin (mTOR) cell signaling complex 1 (mTORC1) (1). The mTORC1 complex is a master regulator of cell growth, making it a high-priority target in cancer and inflammation research (2). Before Raptor can be assembled into the mTORC1, it is first folded by the chaperone protein cytosolic chaperonin containing TCP1 (CCT). CCT is composed of two rings of 8 different subunits, which together form a barrel shape. CCT substrates are folded in the 85 Å wide folding cavity inside the barrel.
The mechanism by which CCT folds many of its substrates has been determined through biochemical and structural biology techniques (3). Raptor, however, is a 150 kDa protein and is too large to completely fit inside the cavity. No folding mechanism has been solved for a protein as large as Raptor.
We propose that Raptor is folded by partially entering the CCT cavity to fold individual domains. This is possibly a multi-step process, where Raptor changes orientation to insert different domains into CCT for folding.
Methods
To solve the structure of the Raptor-CCT complex, we used a combination of cryo-electron microscopy (cryo-EM) and chemical crosslinking coupled with mass spectrometry (XL-MS). Cryo-EM is a technique that allows researchers to average microscopic images together to get 2D images of protein complexes. These 2D reconstructions can then be combined to form a 3D density map of a protein complex. XL-MS is a technique that allows researchers to form crosslinks between proteins and then identify where the crosslinks occur using mass spectrometry. The crosslinks are then used to apply distance constraints to protein models.
To purify the Raptor-CCT complex used in our cryo-EM and XL-MS experiments, we employed a tandem affinity purification procedure. We prepared a Raptor construct with two affinity tags: a hexahistadine tag, which binds metal-chelate columns, and a strep peptide tag, which binds streptavidin columns. The Raptor construct was expressed in HEK 293T cells. The cell lysate was first passed over a Co2+ -chelate column and then a streptavidin column to purify the Raptor construct. This also purified any endogenous CCT that was bound to Raptor in the cell.
For the XL-MS experiments, we used disuccinimidyl suberate (DSS) as our crosslinking molecule. DSS forms 34 Å crosslinks between the peptide backbone of lysine residues. To identify the crosslinks, we used the xQuest software pipeline (4). After three trials, we identified eight crosslinks between Raptor and CCT.
Cryo-EM studies were performed by our long-time collaborator, Jose Valpuesta, in Madrid, Spain. The protein sample used for these studies was prepared in our lab. So far, we only have enough data to generate 2D reconstructions of the Raptor-CCT complex.
Results
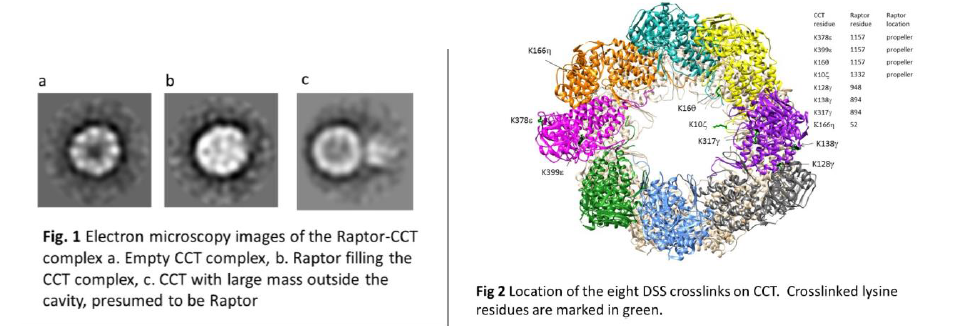
In the 2D electron microscopy reconstructions, we observed two distinct raptor-CCT complexes. In one, Raptor completely fills the CCT cavity and extends out from CCT. In the second complex, we observed that Raptor was attached to the outside of CCT, with the cavity partially filled. (Fig. 1) This suggests that Raptor can be attached to CCT in two different conformations.
In the eight crosslinks between Raptor and CCT, we identified different crosslinks between residue 1157 on Raptor and two separate CCT subunits on opposite sides of the cavity. This is only possible if residue 1157 is in both locations. (Fig. 2)This supports the hypothesis that Raptor has two different conformations when bound to CCT. We also identified crosslinks on the interface between two CCT subunits, suggesting that Raptor passes in between the subunits to exit the cavity instead of exiting out the main opening.
Discussion
Together, the electron microscopy and XL-MS data suggest that Raptor has two conformations in complex with CCT. Raptor partially enters the CCT folding cavity and may pass in between CCT subunits to exit the cavity. This represents a novel CCT folding mechanism and gives insight into how CCT can fold large substrates.
Conclusion
After we have obtained enough cryo-EM data, we will be able to obtain a 3D reconstruction of the complexes. A high resolution 3D image will allow us to accurately dock our Raptor and CCT models together and will clarify many of the remaining ambiguities in the Raptor folding mechanism.
The data from these experiments will allow drug developers to target the mTORC1 complex. They will be able to use the points of contact between CCT and Raptor to develop small interfering molecules that will block Raptor folding as well as its incorporation into mTORC1.
- Betz, C., and Hall, M. N. (2013) Where is mTOR and what is it doing there? J Cell Biol 203, 563- 574
- Laplante, M., and Sabatini, D. M. (2012) mTOR Signaling in Growth Control and Disease. Cell 149, 274-293
- Plimpton, R. L., Cuellar, J., Lai, C. W., Aoba, T., Makaju, A., Franklin, S., Mathis, A. D., Prince, J. T., Carrascosa, J. L., Valpuesta, J. M., and Willardson, B. M. (2015) Structures of the Gbeta-CCT and PhLP1-Gbeta-CCT complexes reveal a mechanism for G-protein beta-subunit folding and Gbetagamma dimer assembly. Proc Natl Acad Sci U S A 112, 2413-2418
- Rinner, O., Seebacher, J., Walzthoeni, T., Mueller, L. N., Beck, M., Schmidt, A., Mueller, M., and Aebersold, R. (2008) Identification of cross-linked peptides from large sequence databases. Nat Methods 5, 315-318
