Carson Storey and Brian Iverson, Mechanical Engineering
Introduction
This project deals with improving the efficiency of thin carbon nanotube (CNT) filters as a means to detect glucose levels in a given solution. It has been shown in previous work that when glucose reacts with oxygen in the presence of glucose oxidase, gluconic acid and H2O2 are formed [1]. When a voltage is applied across a platinum coated CNT filter while in the presence of H2O2, a chemical redox reaction occurs which breaks down the hydrogen peroxide into water and oxygen [2, 3]. During this reaction, electrons are also released and provide a detectable electric current. By means of calibration using solutions of a known glucose concentration, it can then be determined how much glucose is in an unknown solution and, as an example, a diabetic could correct their insulin levels appropriately [4]. However, the concentrations of glucose required to get a measurable output signal are fairly high using traditional sensor architectures. By increasing the surface area, and improving platinum deposition, the signal strength can be increased to detectable levels even from low concentrations.
Methodology
Given their extremely high surface area, CNT filter structures are used as a substrate for platinum deposition to enhance the detectable electric signal. The arrays of CNT micro-channels create more pathways for the platinum to infiltrate the filter, this effect is why the filters are formally referred to as CNT-MMs: carbon nanotube micro-membrane arrays [5]. The increased surface area of the exposed tubes permits more platinum to be deposited, allows more of the peroxide to come in contact with the platinum, and gives a larger measured output current signal for a given concentration.
To further optimize the reaction surface area, three different CNT-MM pore geometries were explored: large diamond, small circle, and large circle micro-channels. A comparison of the respective depths of platinum deposition for each shape is compared in Figure 1. In the early stages of designing these geometries it was decided that the area/volume ratio of void space to CNTs of the different geometries be maintained constant in order to eliminate variables and determine the best pore shape and size. The area ratio was determined to be 70.4% in accordance with previously created CNTMMs and the new pore geometries were calculated in accordance with this ratio.
In order to fabricate CNT-MMs with these respective geometries, masks for photolithography were created by using a combination of AutoCAD and KLayout programs to obtain the proper geometries and layering, respectively. After the CAD design, the mask blanks were laser etched and cleaned in acid leaving the intended geometry. The finished masks were then used in photolithography to pattern several alumina-coated silicon wafers. Once the wafer was evenly covered with photoresist, properly patterned with the mask, developed to remove residual photoresist, and dimensions verified via a microscope, a thin layer of iron was deposited on the mask, covering the remaining photoresist. With a thin layer of iron covering the wafer, the remaining photoresist was removed with an appropriate chemical solvent. In order to cut the wafer to the size needed for the CNT growth furnace, the wafer was coated with a thin layer of protective photoresist and cut into 17mm squares. After dicing the wafers, the protective photoresist layer was removed and the sample was rinsed in preparation for CNT growth. Four samples were placed in the furnace and after varying the time, temperature, and ethylene/hydrogen gas flow rates, CNT-MMs had grown on the silicon wafer and begun separating from it. Lastly, the filters were fully removed from the substrate and oxygen-plasma etched to remove unwanted material that blocked the micro-channels on the underside of the filter. Platinum deposition was then performed statically in a beaker with a magnetic stirring stick to facilitate even platinum distribution across the filter surface.
Results
In the static deposition environment platinum was deposited throughout the CNT-MMs. Figure 1 compares the different geometries of various samples at the center of the channels. The amount of platinum deposited in each of the filters at the center is visually comparable and requires a more rigorous analysis to determine the best pore geometry and size.
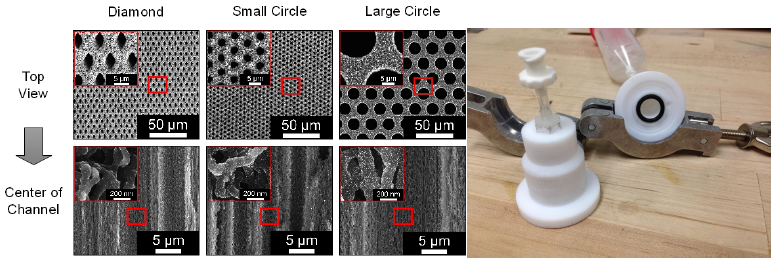
Figure 1: a) CNT-MM shape and depth comparison b)Flow cell deposition set-up (no syringe)
Discussion
AutoCAD and KLayout program functionality was a serious issue in the mask design/creation process. This difficulty led to the creation of one mask with an inverted polarity thereby necessitating a negative photoresist. Negative photoresists are generally more sensitive to development times and often yield less accurate pore sizes and shapes. Regardless, we successfully completed the growth of several CNT-MMs and have begun to characterize the penetration of platinum deposition.
The flow cell itself functions as anticipated, working with both circular as well as the original square samples. More useful data with the flow cell was not obtained largely due to time constraints and the shearing of platinum off the microchannel surfaces. It is suspected this is due to a high fluid velocity in the channels. However, the flow through method was satisfactory inside the porous CNT walls. Although it wasn’t possible to obtain more objective results, the results that were taken from the flow cell were promising. More testing is needed to conclusively determine the optimal pore size/geometry.
Conclusion
Future work in this area includes more extensive testing of the flow cell deposition set-up to determine the optimal cycling times and flow rates. Once the flow cell cycling issue is optimized, it is anticipated that more comparative data will be obtained with respect to the different geometries. In the mean time, the framework for such tests is fully in place with several pore geometries to compare and contrast.
References
- Wang, J., Electrochemical Glucose Biosensors. Chemical Reviews, 2008. 108(2): p. 814-825.
- Montornes, J.M., M.S. Vreeke, and I. Katakis, Glucose Biosensors, in Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications, P.N. Bartlett, Editor. 2008, John Wiley and Sons, Ltd. p. 199-217.
- Claussen, J.C., et al., Electrochemical Biosensor of nanocube-augmented carbon nanotube networks. ACS Nano, 2009. 3(1): p. 37-44.
- Jr., L.C.C. and C. Lyons, Electrode systems for continuous monitoring in cardiovascular surgery. Annals of the New York Academy of Sciences, 1962. 102: p. 29-45.
- Rivas, G.A., et al., Carbon nanotubes for electrochemical biosensing. Talanta, 2007. 74(3): p. 291- 307.
