Benjamin Brownlee and Brian Iverson, Mechanical Engineering
Introduction
The objective of this project was to characterize the increased rate of hydrogen peroxide oxidation using a carbon nanotube microstructure. This is an important step in creating a glucose sensor capable of detecting low concentrations, such as those in saliva. A glucose sensor with this capability would allow people with diabetes to monitor their glucose levels with saliva, eliminating the need to take blood from their finger.
The use of microstructures has been incorporated in a variety of biosensing applications because of their enhanced surface area. Increased surface area improves chemical detection by mass transfer because oxidation occurs at sensing surfaces. As small sensors typically have less surface area, microstructures maximizing the ratio of sensing surface area to volume of solution achieve higher sensitivity.
Methodology
Hydrogen peroxide (H2O2) was used in preliminary sensor testing because glucose oxidizes into H2O2 and gluconic acid. The H2O2 can then be oxidized into hydrogen ions, oxygen and electrons. Oxidation was achieved by passing H2O2 through a catalytic platinum filter constructed with carbon nanotubes. Three different experiments were conducted:
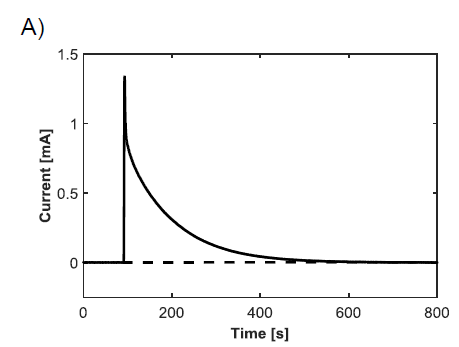
1. An amperometric (current versus time) test used a potentiostat with a three electrode cell to measure the current generated from the oxidation of H2O2. The current versus time data was then integrated to determine the total charge and, subsequently, the concentration of H2O2.
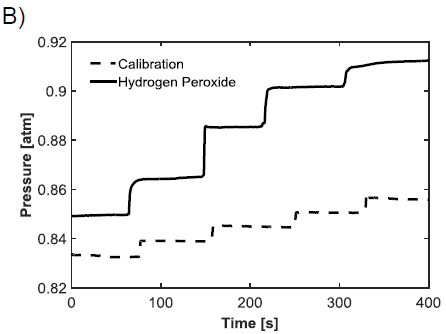
2. A pressure-based experiment used the enzyme catalase to increase the rate of decomposition of H2O2. As small amounts of H2O2 were injected into a sealed container containing catalase, the change in pressure could be related to the oxygen released during the reaction. From this, the concentration of H2O2 could be determined.
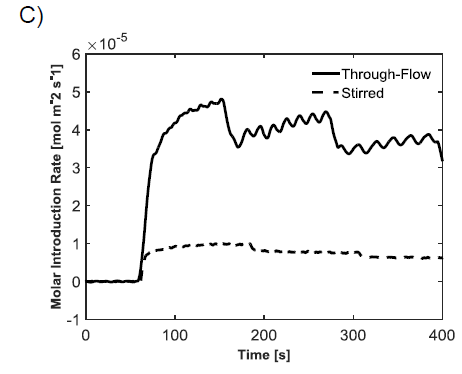
3. A continuous amperometric experiment involved turning the microstructure into the working electrode in the electrochemical cell. This approach enabled continuous measurement of the current produced from the oxidation of H2O2. The current could then be correlated with the concentration of the H2O2.
Results
Example results for each of the outlined experiments can be seen in Figure 1. Consistent measurements of 0.8 moles/liter H2O2 were obtained using the potentiostat. The results obtained from the pressure method were less reliable, but were still close to the amperometric results. Finally, continuous sensing was achieved using through-flow and stirred settings, where the through-flow far outperformed the stirred conditions.
Discussion
Repeated and consistent amperometric measurements showed that the three percent (0.88 moles/liter) bottle of H2O2 was closer to 0.8 moles/liter, likely because H2O2 decomposes naturally into water and oxygen over time. The pressure-based measurements were taken in an attempt to verify the accuracy of the amperometric tests, but the data was not consistent and resulted in a value closer to 0.92 moles/liter. The continuous feedback signal showed the advantage of flowing H2O2 through the microstructure instead of flowing it past the filter in a stirred environment. As can be seen in Figure 1C, the highest through-flow oxidation rate was close to five times that of the highest stirring oxidation rate. The higher current response from this oxidation allows for measuring lower concentrations of H2O2, and thus lower concentrations of glucose. Figure 1C also shows that the oxidation of H2O2 is heavily dependent on the flow rate and stir speed. As expected, faster flow rates and stir speeds result in faster oxidation of H2O2, which can be measured continuously from the microstructure.
Conclusion
The levels of glucose in saliva could be effectively determined with the use of these catalytic microstructures. The sensing of hydrogen peroxide oxidation with these filters should directly correlate with future results involving glucose.
Figure 1: Graphs showing the response of H2O2 oxidation. A) Typical amperometric response to an injection of three percent H2O2 into a conductive solution. B) Pressure results from a sealed container containing catalase with injections of H2O2. The dashed line is an air injection calibration used to determine the concentration of H2O2. C) Comparison of through-flow (solid line) and stirred (dashed line) molar oxidation rates, normalized by surface area. The step downs result from change in flow rate or stir speed. This plot is nearly identical to the response achieved from live feedback from the sensing surface, only the y-axis would display current.