Unrau, Mikkel Boiling and Julie Crockett, Mechanical Engineering
Introduction
The interaction between water-based droplets and heated solid surfaces is one of the most ubiquitous observed phenomena in nature and many industries. Some of the applications include spray coating/cooling, ink-jet printing, lab-in-a-droplet technology, and internal combustion engines. Droplet impingement on Superhydrophobic (SH) surfaces promises great benefits in many of these industries due to the ability of these surfaces to energetically repel water droplets. To adequately determine how SH surfaces might be employed in the applications listed above and many others, I performed a study of water droplets impinging on multiple SH surfaces and over a range of elevated temperature conditions.
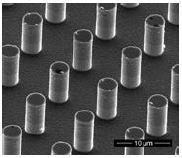
Figure 1: micro-post pattern
Specifically, this project illuminated how SH surfaces affect the boiling behavior of impinging water droplets during spreading and recoiling. Preliminary testing in our lab has shown that the arrangement of the micro-structural pattern on the SH surface plays a significant role in determining boiling dynamics. It was observed that SH surfaces with the micro-posts spaced 8μm apart caused more violent nucleate bubble explosion whereas the SH surface with 16μm micro-post spacing completely mitigated nucleate boiling. These data indicate that boiling regimes can be controlled by the type of surface used in a given application.

Figure 2: (From left to right) Droplet impingement onto a SH surface with 8μm micro-post spacing and a SH surface with 16μm micro-post spacing.
Methodology
To carry out the project my mentor and I defined three variables which would have a significant impact on droplet-surface interaction during impingement: surface temperature, droplet impact velocity, and surface type. The temperatures studied were 150°C, 200°C, 275°C, and 350°C. To change droplet impact velocity, droplets were dropped from 3 heights: 0.75”, 3.5”, and 8”. The surfaces used were varied by their post pitch (P), the distance between post centers in μm, and post height (H), also in μm. The surfaces used were 8P5H (8μm pitch, 5μm height), 8P14H, 16P8H, 16P18H, 16P25H, along with a 16P8H surface that was untreated, rendering it hydrophilic. This last surface was used as a control to verify that our results were in fact due to the superhydrophobicity of our surfaces.
The phenomenon of interest was atomization, which occurs when nucleate bubbles within the droplet burst, releasing a spray of mist. High speed video was taken of each experiment. A Matlab program was developed to count the total number of pixels occupied by atomized droplets and estimate the total atomization. Results were then normalized by the total number of pixels. To allow for accurate comparisons, extreme care was taken to maintain the same lighting, as well as camera placement for each experiment.
Results and Discussion
Based on observations during the experimentation process, the 16P SH surfaces saw little or no atomization. The image processing code confirmed this. The rest of the discussion will therefore focus on the results obtained using the 8P surfaces.
After plotting % atomization and time, some definite trends were noted.
Temperature Dependence: Increasing temperature generally led to more rapid and intense atomization, but there seems to be a temperature above which less atomization happens.
Droplet Release Height Dependence: There appears to be a drop height below which little or no atomization happens. No appreciable amount of atomization occurred at 0.75” drop height. Also, higher drop heights generally show equal or more atomization if other conditions are held constant. One exception of this was the 5H surface at 200°C. For that case, the 3.5” drop height showed more atomization than 8”.
Post Height Dependence: Generally, 5H saw more atomization than 14H. However, this trend is reversed at for the 3.5” drop height at 200°C.
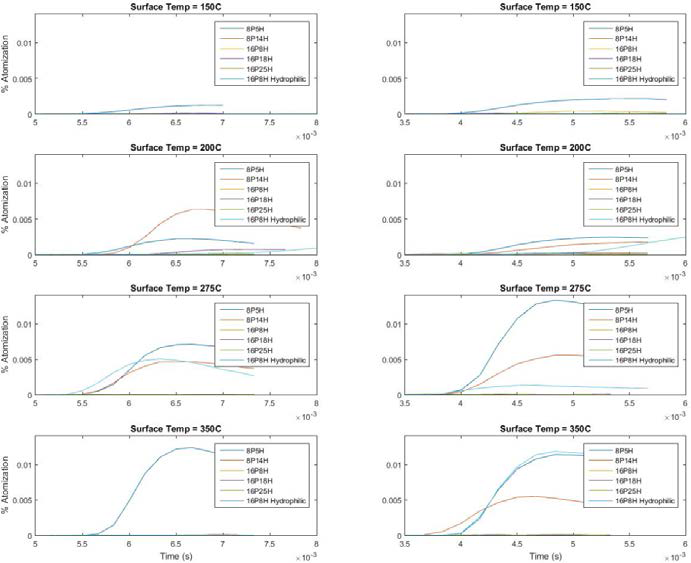
Figure 3: Comparison of 3.5” (left) and 8” (right) droplet release heights at various temperatures. Height of 0.75” not shown due to negligible amounts of atomization detected.
The hydrophilic surface served an important purpose. It is apparent that superhydrophobicity has a strong effect on the interactions between the impinging droplet and the surface. In this case, the SH analog to our hydrophilic surface completely mitigated any atomization.
Conclusion
Experiments were carried out on 6 distinct surfaces to compare droplet-surface boiling interactions. Atomization, caused by nucleate bubble formation, was quantified and compared at three temperatures and 3 drop heights. Little or no atomization occurred in any case for the 16P surfaces. On the 8P surfaces, comparison shows a clear dependence on drop height as well as temperature, with higher values of both contributing to more intense atomization. Post height was also shown to influence atomization. More work must be done to substantiate outlying data from these experiments, as well as to more adequately define the boiling regime on 16P SH surfaces.