Daniel Loveland and Dr. J. Dee Higley Department of Psychology
Original Project Title: Why can’t we all just get along? MAOa genotype variation is associated with aggressive temperaments in nonhuman primates
In place of studying behavior differences among rhesus monkeys with differing MAOa genotypes, my research group and I chose to analyze the effects of genes and the environment on brain chemistry.
Introduction
Variants of the monoamine oxidase A (MAOa) gene are associated with psychopathologies such as depression, anxiety, excessive alcohol consumption, and violence in both humans and rhesus monkeys123. Previous research concerning the MAOa gene has primarily focused on associated behaviors, however this novel study examines genotype and environmental effects (rearing) on specific brain chemistry (through measurements of central monoamine functioning). The brain’s monoamine neurotransmitters are dopamine, norepinephrine, and serotonin, all of which have significant influences on behavior. We tested the hypothesis that MAOa genotypes would interact with maternal presence/absence (AKA, a gene by environment [GxE] interaction) over time to modulate the brain’s monoamine systems: dopamine, norepinephrine, and serotonin.
Methodology
Cisternal cerebrospinal fluid (CSF) was obtained from 116 male infant rhesus macaques on days 14, 30, 90, 120, and 150 of life and assayed for HVA, MHPG, and 5-HIAA (metabolites of the monoamines: dopamine, norepinephrine, and serotonin, respectively, and used to measure levels of these neurotransmitters in the brain). Subjects were reared either as controls with their mothers or in mother-absent, peer-only groups, which introduces the environmental variable.
Results
There were main effects for rearing (p<0.0009, p<0.001) and genotype (p<0.0009, p<0.010) for the norepinephrine and serotonin systems. Repeated measure ANOVAs also showed significant three-way GXE by time interactions for both these systems (p<0.012 and p<0.040 respectively). There was a main effect of genotype (p<0.004), as well as a GXE interaction for the dopamine system (p<0.013).
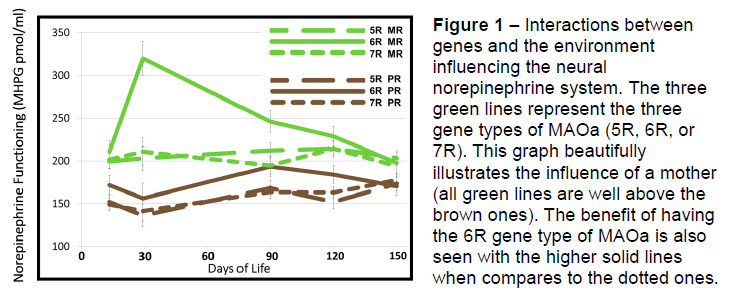
Discussion
These results largely confirmed our hypothesis. As in other studies, our results show a critical role for mothers in the development of the CNS, with peer-reared infants at greater risk for abnormal monoamine functioning, as exhibited by impaired MHPG and 5-HIAA metabolite concentrations4. Interestingly, it was only in the presence of the more functional 6R allele that mother-reared infants showed significantly higher concentrations of the dopamine metabolite HVA (a gene X environment interaction); the other alleles did not interact with rearing for the HVA metabolite. Our findings, along with other studies showing that the transcriptional activity of the MAOa gene is modulated by maternal influences, suggest that there is a “genetic plan” for monoamine maturation; a plan that is modulated by early rearing experiences.5 Most apparent in norepinephrine, our results show that maternal influences can buffer the effects of a less functional genotype on monoamine functioning (mother-reared subjects with less functional 5R or 7R alleles have often significantly higher concentrations of the MHPH metabolite). To the extent that various forms of psychopathology are modulated by the monoamines, our findings show a GxE interaction over time possibly leading to behavioral disorders such as aggression, depression, alcoholism, and anxiety.
Conclusion
Previous studies have tested behavioral differences based on MAOa gene X environment interactions. Our experiment shows the same effects on brain chemistry, presenting possible neurological origins of the psychopathologies observed with MAOa genotypes (e.g., aggression, depression, anxiety, and alcohol abuse disorders). As abnormal neurotransmitter functioning is thought to underlie numerous behavioral disorders, assessing for gene X environment interactions may more accurately explain and predict psychopathological behaviors.
1 Meyer, J. H., et al. (2006). Elevated Monoamine Oxidase A Levels in the Brain: An Explanation for the Monoamine Imbalance of Major Depression. Archives of General Psychiatry. doi:10.1001/archpsyc.63.11.1209
2 Samochowiec J, et al. (1999): Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Res 86:67–72
3 Manuck SB, et al. (2000): A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res 95:9 –23
4 Beaver, K. M., et al. (2013). Exploring the association between the 2-repeat allele of the MAOA gene promoter polymorphism and psychopathic personality traits, arrests, incarceration, and lifetime antisocial behavior. Personality and Individual Differences, 54(2), 164-168. doi: http://dx.doi.org/10.1016/j.paid.2012.08.014
5 Newman, T.K., et al. (2005). Monoamine oxidase a gene polymorphism and infant rearing experience interact to influence aggression and injuries in rhesus monkeys (Macaca mulatta). Biological Psychiatry, 15, 167-172.
