Ashley Nelson and Dr. Scot Steffensen, Psychology
Introduction
The Centers for Disease Control and Prevention attributes approximately 80,000 deaths annually to excessive alcohol use (CDC), and alcohol consumption is the third leading cause of preventable deaths in the United States (Mokdad et al., 2004). These statistics do not even describe the detrimental effects alcohol addiction has on personal lives and families. The accepted model for addiction in the brain is a change in the normal regulation of the neurotransmitter dopamine (DA) in the mesolimbic pathway. Studying this pathway is important in developing treatments for addiction, which has implications to improve the lives of many suffering people. The primary areas involved include the ventral tegmental area (VTA) in the midbrain and the nucleus accumbens (NAc). Dopamine neurons in the VTA are primarily regulated by inhibitory gamma-aminobutyric acid (GABA) neurons also located in the VTA. The GABA(A) receptor on VTA GABA neurons normally allows negatively charged chloride ions into the cell, hyperpolarizing it, which leads to inhibition of the excitability of the neuron. Others have shown in opiate-dependent animals that GABA(A) receptors switch their function from being inhibited by GABA to being excited (Vargas-Perez et al., 2009). We show that the change in DA regulation due to alcohol dependence occurs because of a switch in function of the GABA(A) receptors on GABA neurons in the VTA.
Methodology
Mice and Vapor Chambers
In our experiment GAD GFP mice were housed in ethanol vapor chambers, which allow for monitoring of blood alcohol levels. After two weeks the mice became dependent due to chronic intermittent ethanol exposure in the vapor chambers. We used air-exposed mice from the vapor chambers as controls. Data was collected at maximum withdrawal, which happens 8-24 hours after the last ethanol exposure (Gallegos et al., 1999). GAD GFP mice have an inserted gene that causes GABA neurons to fluoresce under UV light, so GABA neurons can be visualized and distinguished from DA neurons in the slice preparation.
Patch-clamping
We obtained horizontal brain slices from GAD GFP mice containing the VTA. These slices were kept alive in vitro through bathing the brain in artificial cerebral spinal fluid (ACSF) at physiological temperatures (36°C). We used cell-attached patch-clamping to measure firing rates. In patch-clamping, you lower a glass pipette and attach it to a VTA GABA neuron, where you can measure electrical properties of the cell. We recorded firing rates in cell-attached voltage-clamp mode, meaning we measure membrane activity through changes in current by controlling the voltage.
Muscimol administration
The drug muscimol is a known agonist for the GABA(A) receptor, which means that it activates and opens the GABA(A) receptor, mimicking its physiological function. After observing a stable firing rate, we administered a dose of muscimol in the ACSF bath. We used doses of 0.01, 0.1, 1, and 10 μM.
Results
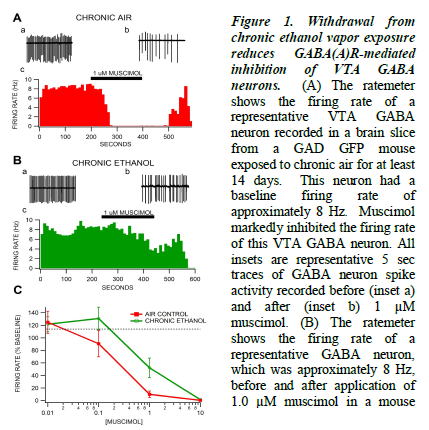 In mice treated in the vapor chambers with chronic air (no ethanol) GABA neurons in the VTA were inhibited by muscimol at doses of 0.1, 1, and 10 μM. This effect is evident by a slowing of the firing rate after muscimol is administered. In mice treated with chronic ethanol vapor, GABA neurons are resistant to muscimol’s inhibitory effects. Instead of slowing, cells maintain a baseline firing rate, and on average even increase firing rate with 0.1 μM muscimol.
In mice treated in the vapor chambers with chronic air (no ethanol) GABA neurons in the VTA were inhibited by muscimol at doses of 0.1, 1, and 10 μM. This effect is evident by a slowing of the firing rate after muscimol is administered. In mice treated with chronic ethanol vapor, GABA neurons are resistant to muscimol’s inhibitory effects. Instead of slowing, cells maintain a baseline firing rate, and on average even increase firing rate with 0.1 μM muscimol.
Discussion
Muscimol activates the GABA(A) receptor, so in the control animals treated with air, we see the normal inhibitory effects of the GABA(A) receptor. However, in the chronic ethanol animals, there is clearly a change in function of the GABA(A) receptor. Activating it no longer inhibits the cells. This effect is important because it shows a clear physiological shift from a non-dependent state to an ethanol-dependent state.
Conclusion
This study has implications to contribute to the general model of addiction and dopamine dysregulation. As we piece together the puzzles of what happens in the brain when a person becomes dependent on alcohol, we will be able to develop better treatments. This change in function of the GABA(A) receptor has the potential to influence the field to investigate GABA regulation of VTA DA cells as the primary gate to an addictive state. One limitation of this study was that we had not perfected our vapor chambers yet. Since the time this data was collected, we have adjusted the ethanol vapor protocol to be more reflective of typical drinking patterns. Future studies include studying the GABA(A) receptor in ethanol-dependent models to see why this functional shift is occurring. Some current hypotheses include both TrkB receptor activation by BDNF and ethanol or endogenous compounds interacting with the GABA(A) receptor.
References
Centers for Disease control and Prevention. (2013). Alcohol and public health: Data, trends, and maps. Retrieved from http://www.cdc.gov/alcohol/data-stats.htm
Mokdad, A.H., Marks, J.S., Stroup, D.F., & Gerberding, J.L. (2004). Actual Causes of Death in the United States, 2000. The Journal of the American Medical Association, 291(10), 1238-1245.
Vargas-Perez, H., Kee, R. T., Walton, C. H., Hansen, D. M., Razavi, R., Clarke, L., Bufalino, M. R., Allison, D. W., Steffensen, S. C., & van der Kooy, D. (2009). Ventral tegmental area BDNF induces an opiatedependent-like reward state in naïve rats. Science, 324(5935), 1732-1734.
