Jessica Jenkins and James Patterson, Department of Chemistry and Biochemistry
Introduction
In many fields of work, such as medicine or pharmaceutical research, it is necessary to separate chemically similar compounds. Liquid chromatography, which involves a mixture of compounds (analyte) dissolved in a mobile phase flowing through a packed column (stationary phase), is the most widely used chemical separation technique. Changes in operational parameters such as the mobile or stationary phase composition, temperature, pressure, pH, etc. affect retention time Operational parameters are currently chosen by trial-and-error because we understand very little about the molecular interactions between the analyte and the mobile phase/stationary phase interface. Specifically, it is unclear how enthalpy and entropy affect retention. If we understood more about the molecular interactions between the analyte and the mobile/stationary phases and how those interactions affect the associated thermodynamic properties, we could better predict optimal separation conditions for medical and scientific purposes. My efforts towards this goal, with help from Dr. Patterson and those in his lab, provided the basis for my Honors Thesis (submitted in July 2014) and Andy Peterson’s Master’s Thesis (submitted in December 2013).1-2
Methodology
For this experiment I modeled a C18 liquid chromatography column by functionalizing a fused silica prism with carbon-18 chains and preparing a sample cell filled with methanol, to mimic an RPLC column. We used a sample analyte, pyrene (shown in Figure 1), to prepare solutions of concentrations between pure methanol and 0.01 mM pyrene in methanol. I chose to examine pyrene because it is a member of a group of compounds that are chemically similar and differ in their physical structure (thus, are difficult to separate using RPLC). It is also chemically unreactive, thus providing a stable experiment. We used a peristaltic pump and allowed the solution to flow at a rate of 0.5 mL/min to promote system equilibration.
To examine the enthalpic and entropic effects involved in RPLC, I and former graduate student Andy Peterson designed an experimental system using second harmonic generation (SHG) spectroscopy to examine the system at the interface between the carbon chains and the methanol solution. SHG is a technique where two photons equal in energy are absorbed to transfer an electron to an excited state; then as the electron returns to the lower energy state, a photon with the combined energy of the two incident photons is released. This light is detected and mathematically related to the concentration of analyte in the mobile phase. We used this setup with a Nd: YAG laser at 532 nm to examine the solutions of pyrene and extract the enthalpic and entropic changes of the system at room temperature.
After examining the solutions at room temperature, I modified the setup to include a temperature controlled water bath and began to repeat the experiment at ~ 7 °C. However, I did not have time to complete the experiment at the colder temperature.
Results and Discussion
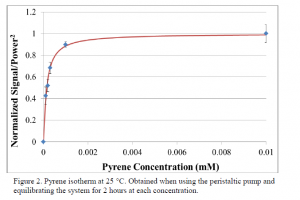
For each run, we began with a system equilibrated with pure methanol, then examined a given concentration, and ended examining 0.01 mM pyrene in methanol. In this way, we normalized the given solution to both pure methanol and 0.01 mM pyrene in methanol in order to compare data collected on various days regardless of minor changes in the system. Each concentration was stored in glass bottles and pumped using the peristaltic pump for two hours to allow complete equilibration. Several data points were collected after equilibration and the average of the points was used to plot the point for each concentration. We collected the room temperature isotherm shown in Figure 2, which fit an absorption retention model and a Langmuir isotherm model. Using thermodynamic equations I extracted the equilibrium constant Keq = 1.6 × 104.
Conclusion
We have created a system that uses a 532 nm laser to produce SHG signal at a model stationary phase / mobile phase interface. This experiment has functioned as a manner of differentiating between concentrations of pyrene in methanol; the initial data obtained has supported the theory that a Langmuir isotherm model fits a system of pyrene in methanol with a C18 stationary phase. Using a peristaltic pump and two hour equilibration time we were able to obtain Keq = 1.6 × 104.
In order to confirm these results and determine the thermodynamic parameters ΔG°, ΔH°, and ΔS° it is necessary to first repeat the room temperature and further explore the cold temperature runs. Once we have confirmed the repeatability of the room temperature Keq, the experiment should be repeated at various temperatures and solvent conditions, first with pyrene and then with the other 4-ring PAHs. We will then be able to determine the thermodynamic parameters and better understand what drives PAH retention. This will be the first step in creating a general model for RPLC analyte retention. Figure 2. Pyrene isotherm at 25 °C. Obtained when using the peristaltic pump and equilibrating the system for 2 hours at each concentration
1. Jenkins, J.L. Molecular-Level Interactions Responsible for Retention in Liquid Chromatography. Honors Department; 2014.
2. Peterson A.D. Nonlinear spectroscopic investigation of adsorption to C-18 model stationary phase. Brigham Young University; 2013. Figure 1. Structure of pyrene.