Dallin L Lindstrom, Bradley W. Hanks, and Professor Brian D. Jensen, Mechanical Engineering
Introduction
Delivering foreign genetic material into human cells to cure or reverse the effects of genetic disease is a challenging task. This process, known as gene therapy, is of the utmost importance in treating many diseases, such as cancer, hemophilia, neurodegenerative diseases, etc. Most of the current gene therapy methods used on culture cells are limited in their effectiveness. This project allows us to determine if the increased conductivity of carbon coated lances results in the delivery of greater amounts of PI. Since DNA is also charged, our technique is a forerunner in using this same process to introduce foreign DNA into cells. The greater the particle delivery efficiency, the greater chance of repairing genetically damaged cells.
Methodology
Lance Array
To fabricate the lance array chips used in this project, micron-wide solid lances (solid needles) are etched into a silicon wafer using MEMS fabrication technology. One 2cm x 2cm silicon chip contains millions of lances. After fabrication, the chip is coated with carbon molecules to increase electrical conductivity.
Cell Culture
HeLa cancer cells were cultured on the bottom of tissue culture plates on a glass slide to ensure uniform injecting surface. The cells were grown in 2 mL Dulbecco’s Modified Eagle Medium (DMEM) with Fetal Bovine Serum (FBS) and gentamicin. In each well, HeLa cells adhere to the glass slide and are cultured for 24 hours prior to injection.
Injection Process
It has been shown in previous studies using the same protocol that 80 microliters of PI maximizes the uptake when silicon lance arrays were used for nanoinjections (reference). For one round of injections, four six-well plates are used. Three types of samples are needed for each test: negative controls (no PI and no injection), positive controls (PI added but no injection), and injected samples (PI added and injected). The controls give us baselines to measure against for PI uptake in injected samples. The carbon coated lance array chip is mounted to an injection device. Employing compliant springs, the injection device allows the lances to be pushed into the culture of cells and released easily. Three different protocols were conducted to test the efficacy of the carbon coated lance arrays. The first test relies on diffusion. Lances are poked through the cell membrane and left inside for 5 seconds before being drawn out. The PI in the surrounding solution diffuses into the cell through membrane pores opened by lances. For the second test, a constant DC voltage is applied to the lances. Negative 1.5 VDC attracts the positively charged PI particles for 20 seconds. The lances are then pushed through cell membranes and the voltage is reversed, repelling PI particles into the cells. After 5 seconds, the lances are removed from the cells. The third method is identical to the second, except for the addition of a short pulse immediately after penetrating cell membranes with the lances. Ten pulses between 1.5 V and 5 V are applied within 20 milliseconds. The short pulse gives a stronger repulsion force without the toxic effects of a higher voltage on cells. Each protocol was conducted with both a carbon coated chip and a bare silicon chip so that data from each set of injections can be compared to determine the effect the carbon coating has on our injection efficiency.
Flow Cytometry and Analysis
After the injection process, trypsin is added to each well, and plates are incubated for 5 minutes to allow cells to detach from the bottom of the well. DMEM is added to deactivate the trypsin, and the contents of each well are added to separate FACS tubes and centrifuged to allow the cells to congregate at the bottom of each tube. The contents of each tube are run through a flow cytometer. PI glows when bound to genetic material, and the cytometer reads this fluorescence to discriminate between PI positive and PI negative cells. Flow cytometry data is then analyzed to determine what percentage of cells expres
Results
Viability of Cells
The greatest difference in viability between positive controls and injected samples came in the pulsed injections, with about a 10% difference. The 1.5V injections showed about an 8% difference and the diffusion samples only showed very little difference.
PI Uptake
PI uptake was measured against positive controls. Table 1 shows the uptake of both positive controls and injected cells. All injected samples were mostly similar between the carbon coated and silicon lanced injections. Diffusion showed a 20% difference between the positive controls and the injected samples, the differences in the silicon chip and the carbon chip being reflected in both the silicon chips and carbon chips. The 1.5V samples showed a 23% difference between the positive controls and injected samples. Pulsed injections exhibited a 40% difference between the positive controls and the injected samples.
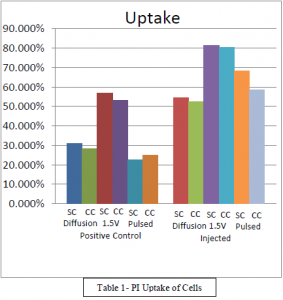
Discussion
There was little difference between the effect of diffusion and 1.5V injection methods. The data shows, and it can be seen in Table 1 that there is little difference between the uptake percentage of silicon and carbon coated chips. It would seem then that there is no benefit of increased conductivity of the lances when performing PI injections. There is apparently a threshold that is reached where there can be no more benefit of increasing the conductivity through the lances. It could be that the lances are saturated with PI completely and increasing the conductivity would make no difference because there wouldn’t be additional space for more PI anyway.
Conclusion
Increasing the conductivity of the lances makes no difference in the percentage of cells that are PI positive. Carbon coated lances and silicon lances are similar in the ability they have to introduce PI into HeLa cells.
