Rupak Bajagain and Dr. Morris Argyle, Chemical Engineering
Introduction
The purpose of this project was to develop water gas shift catalysts that are more stable and active than the existing ones. Stable catalysts last longer and are more efficient, which is important to economics and to gain widespread application. Currently the demand for hydrogen as a fuel is low but in the future there is a high potential that hydrogen could be important in world energy. This project was performed by a group of undergraduate and graduate students from BYU. Most of the work in the project was performed by group members. The report focuses mainly on the tasks that are completed to develop the active catalyst, which includes designing the reactor, catalyst preparation, performing kinetic experiments and evaluation.
Catalyst Synthesis
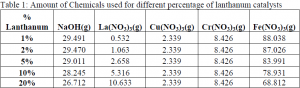
Our goal was to develop high temperature water gas shift catalysts which are stable. The co-precipitation method was used for the preparation of catalysts. Water-soluble chemicals, such as iron nitrate (Fe(NO3)3), chromium nitrate (Cr(NO3)3), and copper nitrate (CuNO3) were dissolved in water. Traditionally, a fourth component, such as aluminum nitrate (Al(NO3)3), is added to test the effectiveness of this component in stabilizing the catalysts and making them more active. In this case, we used lanthanum nitrate La(NO3)3 to test the effectiveness in stabilizing the catalysts and making them more active. NaOH, a base, was added to increase the pH to about 10 to precipitate in hydroxide form. The precipitate was washed with purified water, filtered, and dried to form a powder. The dried powder was then “calcined”, which is a thermal treatment in air or more dilute oxygen to put the catalyst components into a relatively stable oxide form. During drying and calcination, large amounts of unwanted soluble ions and water were removed from the system. The catalysts were pelletized and then crushed until their size is between 125 m to 250 m. The catalysts were then stored in separate vials. The catalysts are now ready for reaction. The following table shows the amount of different chemicals used to achieve different percentages of lanthanum in the catalysts.
The following reactions describe the formation of the mixture of iron, chromium, copper, and lanthanum oxides.
Fe(NO3)3 + 3NaOH→Fe(OH)3 + 3NaNO3 2 Fe(OH)3 →Fe2O3 +3H2O(upon heating)
Cr(NO3)3 + 3NaOH→Cr(OH)3 + 3NaNO3 2 Cr(OH)3→Cr2O3 +3H2O (upon heating)
Cu(NO3)2 + 2NaOH→Cu(OH)2 + 2NaNO3 Cu(OH)2 → CuO +H2O (upon heating)
La(NO3)3 + 3NaOH→La(OH)3 + 3NaNO3 2La(OH)3 →La2O3 +3H2O (upon heating)
Kinetic Experiment
The first step is to reduce the catalyst. This was done by flowing argon, hydrogen, and water in a heated reactor for two hours at 400°C. Then we ran the reaction in a tubular reactor with about 0.1 g of catalyst. Water (H2O) and carbon monoxide (CO) were used in a ratio of 3:1. The reaction was operated at 400°C. This process did not produce any hydrogen gas. We tested other catalysts with the same amount at same reaction condition. We were not successful to get any hydrogen gas. Then we decided to change the reduction process making the ratio of (CO+ H2): (CO2+ H2O) equal to 1.4. After reducing the catalyst at new condition, the reaction was operated at same condition. Various samples were analyzed by gas chromatography, but none of the samples produced any hydrogen gas.
After several attempt to produce hydrogen, we decided to increase the conversion by increasing the amount of catalyst used in the reaction. We first used 1 g instead of 0.1 g of catalyst. In the beginning, no hydrogen was detected, but it produced carbon dioxide gas. Since carbon dioxide is one of the products of the reaction, we expected hydrogen was also being produced, so we ran the reaction continuously for another day. The sample of the product gas analyzed on next day by gas chromatography shows hydrogen was produced. So, finally hydrogen was produced after a long effort.
Evaluation of Improved Catalyst
From the research, we have found out that the lanthanum catalyst takes time to become active. But the relative performance of each catalyst has not been performed yet due to lack of time. Evaluation of catalyst is the next step of the research that is going to be performed after New Year. Evaluation of each catalyst takes 5-6 days. So, it takes about a month and half to evaluate all of the catalysts. Based on this result, the effectiveness of the amount of lanthanum addition and the amount of other components will be determined.
Design of Reactor and Future Work
Designing the reactor was an important part of the project. We had to move our laboratory to different room due to ventilation restrictions in the basement of the Clyde Building, which delayed our plan to finish the construction from February to July 2010. This delayed performing kinetic experiment. Although our experiment process was delayed, we got positive results by the end of December 2010. The next step will be evaluation of effectiveness of each catalyst relative to each other.
Overall, the research was very helpful in applying all the theoretical knowledge that I have gained so far into the real world.
