Sarah Bahr and Dr. Emily Bates, Department of Chemistry and Biochemistry
Introduction
Migraine is a disorder that can include aura, headache, nausea, and hypersensitivity to all sensory modalities. It affects over thirty million people in the US. Migraines affect men and women equally until puberty, but overall migraines affect 3x more women than men. They also increase in frequency and severity during periods of estrogen fluctuation and often subside after menopause. Moreover, hormone supplements and birth control can affect migraine patterns. These correlations of migraine episodes to the menstrual cycle and increased pain sensitivity in response to estrogen suggest that differences in estrogen signaling contribute to migraine. We hypothesized that estrogen receptor β (ERβ) is involved migraine pathogenesis. We determined whether deletion of ERβ causes decreased pain sensitivity in mice after a migraine trigger. To do this, we injected ERβ knockout mice with a common migraine trigger, nitroglycerin. We then determined thresholds for detection of thermal stimulus. In addition, we have measured thresholds to induce aura in ERβ knockout mice. Our preliminary results indicate that ERβ knockout mice do exhibit decreased sensitivity to thermal stimuli compared to wild type siblings after administration of nitroglycerin. This confirms our hypothesis that estrogen receptor signaling is part of migraine pathogenesis.
Methodology
Nitroglycerin was used as a migraine trigger to induce migraines in mice. Nitroglycerin has been proven to induce migraines. We then performed a thermal nociception assay, using a hargreaves machine, on wildtype ERβ, heterozygous and homozygous ERβ knockout mice. Differences in response latencies to thermal stimuli allowed us to determine differences, if any, between genotypes and their associated thermal hypersensitivity. If ERβ is involved in migraine pathogenesis, a decrease in hypersensitivity should be observable in ERβ knockout mice.
Results
Discussion
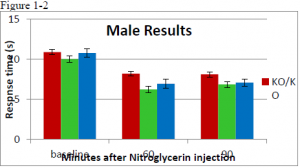
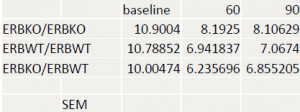
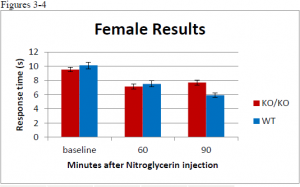
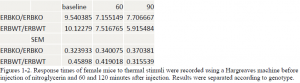
There was no statistcal difference between wildtype or ER knockout mice before injection. In female mice, there was a significant difference at the 90 minute time point between wildtype and ER knockout mice (ttest= .00085) but no difference at the 60 minute time point. In the male population there was a significant difference at the 60 minute time point (ttest= .03617). At the 90 minute time point the difference was significant at a 90% confidence interval, but not at 95% (ttest = .06944). A difference between male and female was expected.
Conclusion
These results strengthen the role of ERs in migraine pathogenesis. Our results indicate that estrogen signaling is involved in migraine pathogenesis. The prescence of estrogen receptor beta results in increased sensitivity to migraines. The absence of this receptor results in decreased pain. This appears to agree with studies showing that migraines occur more commonly in women than in men. Interestingly, female mice appeared to be overall more sensitive. This also concurs with studies that have found that estrogen is involved in pain signaling in the body.