Stephen Wood and Dr. Brian Mazzeo, Electrical and Computer Engineering
The interaction of proteins with other molecules is a common phenomenon that is relevant to various disciplines such as medicine and material science. There are several aspects regarding these molecular interactions that can be researched with one of several measurement techniques. Dielectric spectroscopy is an inexpensive and non-invasive technique used to probe the electrical properties of materials. It was developed in the early twentieth century as a method to measure the change in the dielectric constant of a material in response to changes in frequency. The change or relaxation in the dielectric constant as a function of frequency allows for the calculation of the dipole moment and the effective radius of a large molecule or aggregate. In this study, dielectric spectroscopy was used to quantify the interaction of avidin and biotin. Biotin is a ligand that binds with avidin to form a structure. In theory, a titration experiment can be performed to determine the saturation limit of biotin that binds with a given amount of avidin.
Because the proteins are the most expensive part of this investigation, it is desirable for the measurement cell to be as small as possible. A 60 μL Teflon cell with stainless steel electrodes was attached to a 4294A Agilent impedance analyzer. The impedance analyzer measures the parallel capacitance and conductance of the system, and using these two values the permittivity and conductivity of the solution can be extracted. Sweeps were made from 40 Hz to 110 MHz, and the cell was calibrated using air and 0.1 mM HCl. Protein solutions were mixed using avidin (A9275) and biotin (B4501) from Sigma-Aldrich in 0.1 mM HCl. After calibration, 60 μL of 1 mg/mL avidin in 0.1 mM HCl was pipetted into the cell and frequency sweeps were taken with the impedance analyzer. The solution was then evacuated from the cell, rinsed with DI water, and replaced with a 2 mg/mL avidin solution to ensure linearity. Afterwards, 1 mg/mL biotin in 0.1 mM HCl was titrated in 1 μL increments. The permittivity and conductance of the solution was monitored throughout the experiment to ensure stability.
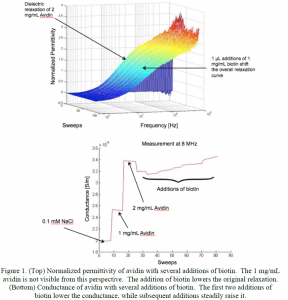
Figure 1 shows preliminary permittivity and conductance results of the avidin-biotin binding. The initial avidin measurements clearly show a relaxation in the permittivity. The addition of biotin reduces the relaxation, and after several additions of biotin a saturation point is reached when the available binding sites are occupied and the dielectric relaxation does not change. It should be noted that equal additions of biotin do not result in equal decrements of the dielectric relaxation. This is due to the fact that changes in the conductivity affect the electrode polarization that occurs at low frequencies, which thus affects the measured permittivity. When biotin is first added to avidin, a distinct decrease in the conductance occurs, after which the additions of biotin lead to a steady increase in the conductance. Previous research has shown that biotin bound with avidin has a lower dipole moment than isolated avidin and would thus lead to a lower conductivity. The increasing conductivity in the figure denotes an increasing number of free biotin molecules in the solution following the occupation of the binding sites.
These preliminary results promise two reference points to quantify the extent of the molecular interactions. Repeatability and quantitation have not yet been established, thus disallowing an accurate calculation of the dipole moment and effective radius of avidin bound with various amounts of biotin. Despite this, the raw measurements are promising results.
These experiments show that dielectric spectroscopy can serve as an effective tool to measure small structural changes in molecules. The importance of factoring conductance into the quantification of these molecular interactions is shown to be crucial, as the permittivity measurements alone do not suffice. More sensitive biosensors can be developed using these techniques and can play a vital role for new drug discovery.