Kayleen Thompson and Dr. Merritt Andrus, Chemistry and Biochemistry
Englerin A, which comes from an East African plant, Phyllantus englari, has been found to be an impressive anti-renal cancer agent. It is even more promising than another leading natural anticancer agent, Taxol.1 Englerin A has a unique, complex structure, and its exact mode of action is currently unknown. The key features of englerin A are the cinnamate ester and glycolate ester with an intervening lipophilic cyclic hydrocarbon core. The englerin structure can be effectively mimicked with a more simplified core structure. For this project, I worked on synthesizing an analog of the anticancer agent (-)-englerin A that contains key groups and stereochemistry of the agent, but has simplified core structures.
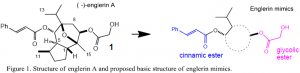
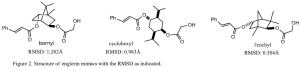
The first step was to select structural mimics based on calculations using molecular mechanics (MMFF94, Spartan, Wavefunction). The key features of englerin A that we included in our mimics are the cinnamate ester at C6 and a glycolate ester at C9 with an intervening cyclic hydrocarbon core (Figure 1). The lipophilic core structure holds the cinnamic and glycolic esters together with the same approximate distance and geometry constraints as englerin A. Molecular modeling calculations were performed to assess the ability of the proposed core structures to keep the structural geometry of englerin A. The mimics we chose to synthesize include a cyclohexyl mimic, a benzyl mimic, and a fenchyl mimic (Figure 2). The root mean square deviation (RMDS) from englerin A is indicated in the figure for each analog.
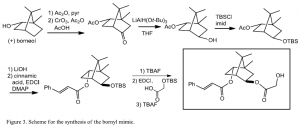
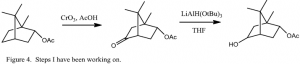
I have been working on synthesizing the bornyl mimic, the plan for which is outlined in Figure 3. The first step is a remote oxidation of (+) borneol using chromium trioxide, as documented by Allen et al.2 Since this was a reaction I had never attempted before, I first tried the reaction starting with (-)- bornyl acetate, as shown in Figure 4. After several tries, following the procedure indicated by Allen et al., the highest %yield I was able to obtain after column purification was only 6.9% for the first reaction. Obviously, the first reaction needs to be optimized to give a higher yield. The next step (a reduction) generated the desired alcohol with a 20% yield. This step also needs to be optimized to give a better yield.
There is still a lot that needs to be done with this project. Each step, particularly the first one, needs to be optimized to give the best yield and best purity. Furthermore, the synthesis of the bornyl mimic needs to be completed. The synthesis of the other two mimics also needs to be attempted. Once this has been done, the next step will be to perform cell assays to test the potency of the mimics. The results obtained from these assays will be used to make further modifications of the englerin mimics.
References
- Ratnayake, R.; Covell, D.; Ransom, T. T.; Gustafson, K. R.; Beutler, J. A. Englerin A, a selective inhibitor of renal cancer cell growth, from phyllanthus englari. Org. Lett. 2008, 11, 57-60.
- Allex, M.C.; Darby, N.; Salisbury, P.; Sigurdson, E. T.; Money, T. Chemical and microbiological remote functionalisation of (+)- and (-)-bornel acetate. Can. J. Chem. 1979, 57, 733-741.
