Jared Huber and Dr. Gregory F. Burton
GOAL
Determine the pathway by which FDC cytokines activate HIV transcription in CD4+ T‐cells harboring latent HIV.
BACKGROUND & IMPORTANCE
HIV Latency in CD4+ T Cells
Human Immunodeficiency Virus (HIV) attacks the immune system by infecting helper (CD4+) T‐cells. HIV injects viral genetic material into the T‐cell, and then integrates the viral genome into the host cell DNA. Infected CD4+ Tcells either begin replication of new virions, or enter a resting state where the HIV is latent or non‐replicating. The virus remains in latency until the cell is reactivated by outside signals and begins to produce virus. The latent reservoir in T‐cells is untreatable with current antiretroviral therapy (HAART), which only destroys free viral particles. If therapy is stopped, T‐cells harboring latent HIV can be activated, and quickly produce enough infectious progeny to return infection to pre‐treatment levels. Ultimately, the immune system is destroyed and death soon follows.
FDC & Latent Virus Transcription
Follicular Dendritic Cells (FDCs) reside in secondary lymphoid tissue and play a critical role in immune responses. They initiate HIV transcription in activated CD4+ T‐cells by producing TNF‐alpha, which acts through the NFκB pathway (Thacker, et al 2009).
Cytokine Signaling
Cells in the immune system activate in response to extracellular signals such as antibodies or cytokines (molecular messages) that bind to receptors on the target cell’s surface. The binding triggers a response in the cell, similar to the flipping of a light switch.
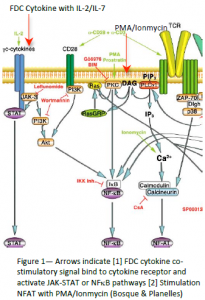
According to Bosque & Planelles, extracellular signals (CD3/CD28 antibodies) turn on resting CD4+ T‐cells through the NFAT signaling pathway (figure 1) at levels of 45% activation. However, when Tcells were treated with a secondary signal (PMA & Ionomycin) they showed small, but significant, activation. Prompted by that observation, researchers in Dr. Burton’s laboratory hypothesized that FDC‐cytokines would further enhance T‐cell activation and virus production. Because FDCs typically provide secondary or costimulatory signals, a weak primary signal (i.e. IL‐2/IL‐7) was used in conjunction with FDC‐cytokines. Results indicate that FDCcytokines and primary signal achieve 35% viral activation. In contrast, stimulation with only IL‐2/IL‐7 resulted in 5% activation. However, IL‐2/IL‐7 do not activate T‐cells through the NFAT pathway, but through the JAK‐STAT or NFκB pathways (fig. 1). In other words, FDC‐cytokines can turn on the light bulb of HIV activation by flipping different switches, or going through the JAKSTAT pathway or the NFκB pathway. By identifying the signaling pathway involved, we lay the groundwork for potential pharmaceutical intervention for the eradication of the latent HIV reservoir in T cells, and may elucidate an actual site in vivo where latent virus reactivation can occur.
PROPOSAL
Hypothesis: FDC‐cytokine(s) with IL‐2/IL‐7 activate latent HIV in CD4+ T‐cells via the JAK‐STAT or NFκB pathways.
Experimental Procedure: In order to create a model of resting CD4+ T‐cells harboring latent HIV, we will obtain Peripheral Blood Mononuclear Cells (PBMCs) from normal donors and infect these cells with envelope (env) deficient HIV, which are capable of only a single round of infection. This ensures that no infectious progeny are produced and only latently infected cells survive (Bosque & Planelles).
[1] Establish Latent Infection in CD4+ T‐cell: Day 1—Obtain PBMCs from healthy donors. Isolate CD4+ T‐cells with Magnetic‐Activated Cell Sorting (MACS) microbead‐negative selection. Culture cells in complete culture media. Day 3‐‐Add IL‐2 (30 IU/mL) to culture. Day 7—Infect cells with env deficient HIV via spinfection method. Day 14‐ 21—Culture cells in complete media to select for cells with latent virus (Bosque & Planelles, 2009).
[2] Isolation of FDC: Cut human tonsils into small pieces and incubate with blendzyme and DNAase to release cells from the tissue. The single cell suspension is subjected to Percoll gradients. Wash low‐density cellular portion (contains FDCs) in RPMI and put cells into culture media. Label cells with antibody and separate by Fluorescence Activated Cell Sorting (FACS) (Thacker, 2008). Culture FDCs and isolate supernatant. Supernatant contains soluble factors—including cytokines—that activate resting T‐cells.
[3] Activation Assay: Day 14 cells are incubated with primary signal IL‐2/IL‐7 and FDC supernatant.
[4] Inhibition of JAK‐STAT Pathway: 2 hours prior to activation assay, cells are incubated with Leflunomide (125 ng/mL) (inhibitor of JAK‐STAT) pathway, Wortmannin (250 nM) (inhibitor of NFκB pathway), both inhibitors together, and Cyclosporin A (CsA) (500 ng/mL) (inhibitor of NFAT pathway).
[5] Flow Cytometric Analysis: Levels of activation are determined by FACS analysis of p24 (Bosque & Planelles). Cells treated with Leflunomide should exhibit baseline levels of activation. If cells cultured with Wortmannin do not demonstrate decreased activation, T‐cells are activated only through JAK‐STAT. If activation decreases, the NFκB pathway is employed. CsA should not inhibit activation and functions as a positive control.
[6] Mass Spectrometry: FDC supernatant analyzed with Mass Spec to identify cytokines that activate latent virus.
EXPECTED OUTCOMES
Results of the experiment will be published in a scientific journal such as Journal of Virology or Journal of Immunology. Also, results will be presented at the CPMS Spring Research Conference, and submitted as a final report for Chem 497R (research for credit).
QUALIFICATIONS
I have worked in Dr. Burton’s laboratory for nearly three semesters, and am experienced in the materials and methods, specifically the culture of CD4+ T cells that harbor latent HIV and FDC isolation. I have assisted other students on similar projects. I am currently enrolled in Chemistry 497R for the second semester. Dr. Burton is an expert in Immunology, especially as it relates to FDCs and HIV. He has published numerous articles on the role of FDCs as a reservoir for latent HIV, as well as the interaction of FDCs and the latent T cell reservoir. This past spring he received an NIH grant for his research.
PROJECT TIMETABLE
November‐December 2010: Establish latent HIV in T cells; Isolate FDCs; Mass Spec analysis; Receive FACS training. January‐March 2011: Analyze levels of reactivation with FACS; Repeat needed experiments; April‐June 2011: Compile data and prepare for publication and presentation.
RELATIVE LITERATURE
- Bosque A, Planelles V, Induction of HIV‐1 latency and reactivation in primary memory CD4+ T cells, Blood, 1 January 2009, Vol. 113:1, p.58‐65
- Thacker TC, Zhou X, Estes JD, Jiang Y, Keele BF, Elton TS, Burton GF. Follicular Dendritic Cells and Human Immunodeficiency Virus Type 1 Transcription in CD4+T Cells. J. of Virology, Jan. 2009, p. 150–158
