Brandon Gassaway and Dr. Emily Bates, Department of Chemistry and Biochemistry
Anderson-Tawil Syndrome (ATS) is characterized by morphological defects including clinodactyly (abnormal curvature of digits), syndactyly (fused digits), micrognathia (small jaw), cleft palate, and hypertelorism (wide-set eyes) as well as periodic paralysis and heart arrhythmia. Mutations in inwardly rectifying potassium (Irk) channels are found in patients with ATS. Expression of mutant Irk channels causes similar physiological and morphological defects in mice.1
Irk channels are transmembrane proteins consisting of four subunits that coordinate together to form an ion conduction pathway across the cell membrane. The genetic mutation in Irk channels that causes ATS creates a dominant negative product, or a protein that lacks or contains a disrupted functional domain, but is still able to form the tetramer.1 The presence of one dominant negative subunit in the tetramer may be sufficient to alter proper ion conduction,2 and causes the paralysis and arrhythmia characteristic of ATS.
Periodic paralysis and heart arrhythmia are logical consequences of mutations in Irk channels that regulate ion flow across cell membranes and neuronal membrane excitability3. The morphological defects observed, however, were surprising because they suggest a previously unknown link between membrane excitability and development. Previous data using knockout animals suggest that disruption of Irk 2 causes defective wing phenotypes. This project attempted to genetically rescue that phenotype by ectopic expression of wild type Irk 2 in the knockout animals.
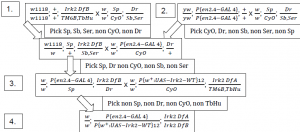
We expect that if loss of Irk 2 is responsible for the defects observed, expressing Irk 2 ectopically in the wing will restore normal wing patterning to the flies and reduce the number of observed venation defects. The appropriate flies require Irk 2 knockout (Df (3R) Irk 2+/Df (3R) Irk 2 exel 6194{w[+rC]=xp-U}exel 6194, or Irk 2 DfA/Irk 2 DfB), the Irk 2 WT p-element (UAS Irk 2 WT), and a transcriptional activator on the wing promoter were generated from our current stocks to show definitively that Irk 2 is responsible for the defects. Chromosomes are marked so that the correct genotype fly can be selected. Marked chromosomes are designated Sp, CyO, Dr, Sb, and Ser. The procedure is outlined in the following scheme:
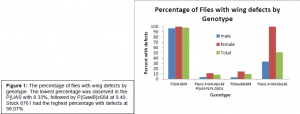
The non Dr, non CyO, non Sp, non TbHu offspring from 4 (male and female) were characterized by percent survival and morphological defects in the wings. These offspring were compared to the knockout flies (stock 99) and Berlin white (stock 1) flies as positive and negative controls. If our hypothesis that reintroducing wt Irk 2 rescues the Irk 2 deficiency phenotype, then these offspring should behave more like the Berlin white flies than the knockout flies.
The first time this experiment was performed, we discovered that the driver being used (MS 1096) caused defects in the control (which consisted of the driver without the UAS-Irk 2 wt element) that masked the defects caused by the Irk 2 deficiency. Because of these defects, reliable data could not be obtained and a new driver needed to be found. Of the several drivers that were commercially available, we selected the P{en2.4-GAL4}e16E P{UAS-FLP1.D}JD1 driver because it caused fewer defects then most of the others, and was on chromosome 2, making a cross to Irk2 DfA/Irk2 DfB strains possible. Irk 2 is on chromosome 3, thus if the driver we needed was also on chromosome 3, we would have had to look for recombination between the chromosomes in order for both the Irk 2 deficiency and the driver to be on the same chromosome. The flies that are published to drive expression of Gal4 in the wing were also crossed to UAS-GFP animals to ensure Gal4 expressed specifically in the developing wing. GFP fluorescence was detected by dissecting the imaginal wing disc from the larva. This experiment is now being repeated with the new driver.
References
- Plaster, N.M. et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell 2001, 105, 511–519.
- Bichet, D.; Haass, F. A.; Jan, L. Y. Merging functional studies with structures of inward-rectifier K(+) channels. Nat. Rev. Neurosci. 2003, 4, 957–967.
- Choe, S. Potassium channel structures. Nat. Rev. Neurosci. 2002, 3, 115–121.