Marie Killian (Chilton) and Dr. Jaron Hansen, Department of Chemistry and Biochemistry
Organic peroxy radicals react to form tropospheric ozone, which harms the health of humans and vegetation and is considered the most significant pollutant in rural areas.1 Many of the individual reactions in the overall mechanism for ozone formation involve radicals. Radicals are highly reactive and therefore it is challenging to measure their fundamental properties directly. Particularly, it is difficult to determine an accurate reaction rate constant for reactions involving radicals. This rate constant is largely dependent on the atmospheric conditions. Dr. Jaron Hansen is currently studying the effect of water vapor on the kinetic rate constant for the reaction of 2-hydroxyethylperoxy radical with itself. This research explored two methods for improving the determination of the reaction rate constant of radical-radical reactions from experimental data. Method 1 was tested on the self-reaction of HO2, the most abundant peroxy radical in the atmosphere. The kinetics of HO2 have been widely studied and therefore it was a good test for the analysis method. Method 2 was applied to the reaction of 2-hydroxyethylperoxy radical with itself.
Method 1
The instruments used to collect the data can significantly affect the experimental determination of kinetic properties. The delay between the actual event and the instrument’s record of that event can be particularly significant. A paper published earlier this year2 presented a method for simultaneously determining the time delay of a calorimeter, the kinetic rate constant, and other properties of the reaction of interest. The paper further suggests that this method could be extending to other types of data. In this research, we applied this model to data collected on the self-reaction of HO2. Although the method simultaneously fit for the parameter of interest, it failed to accurately fit the entire curve, as shown in Figure 1. In particular, the fit failed at the first data points and therefore did not capture the steepest changes in signal, resulting in significant error in the kinetic rate constant. 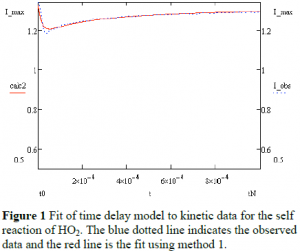
Although this method appears to account for the time delay of the instrument, the model fails to fit to the data. The data shown in Figure 1 is the intensity of a particular wavelength of light transmitted through the reaction cell. This intensity is related to the amount of light absorbed by species in the cell, which is in turn related to the concentration of molecules in the cell that absorb at this wavelength of light. This method depended on the assumption that during this time only the HO2 radical was absorbing the light and that it was only reacting with itself. As demonstrated in Figure 1, the data failed to fit to this first order reaction model, suggesting that other molecules are also absorbing in this time frame and that HO2 may be reacting with other molecules, resulting in a more complex reaction scheme which could not be supported using Method 1.
Method 2
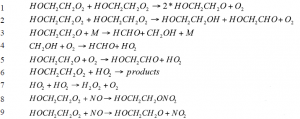
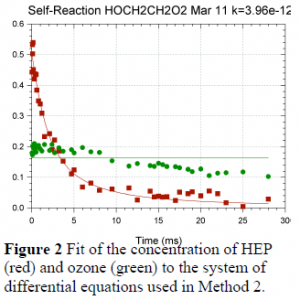
In order to account for the secondary chemistry occurring in the reaction cell, we fit the data to a system of differential equations describing the reactions that could affect the measured signal. This method was used to analyze data collected for the 2-hydroxyethylperoxy (HEP) radical self-reaction. In this experiment, ozone and HO2 were also formed during the experiment and absorb in the same wavelength range as HEP (220-260 nm). Therefore, the model needed to account for the reactions involving these species as well in order to accurately fit the data. Below are the reactions used in the model.
These reactions led to a system of differential equations describing the change in the concentration of each species. As shown in Figure 2, this model successfully fit the data to both the change in concentration of HEP and ozone. Once fit to the experimental data, this model determined the observed rate constant for the HEP self-reaction (reaction 1).
Conclusion
Method 2 is being used to measure the effect of water vapor on the rate constant for the HEP self-reaction (reaction 1). Similar models will be implemented to fit data measuring other reactions, particularly the reactions of HEP with HO2 and HEP with NO. These measurements will improve atmospheric modeling of the effects of particular emissions
References
- Kumar, U.; Prakash, A.; Jain, V. K. A Multivariate Time Series Approach to Study the Interdependence among O3, NOx, and VOCs in Ambient Urban Atmosphere. Environ. Model. Assess.2009, 14, 631-643.
- Hansen, C.W.; Hansen, L.D.; Nicholson, A.D.; Chilton, M.C.; Thomas, N.; Clark, J.; Hansen, J.C. Int. J. Chem. Kinet. 2011, 43, 53-61.