Eric Bready and Dr. Merritt Andrus, Department of Chemistry and Biochemistry
The purpose of this project was completion of the total organic synthesis of the anti-cancer compound (S)-equol. My goal was to carry out our planned synthesis of this molecule using Phase Transfer Catalysts, (PTCs), which promote the formation of the S enantiomer as opposed to the R enantiomer of equol (S and R enantiomers are like right and left hands, which are mirror images of each other—in organic synthesis usually only one form is desired) (1). In using PTCs it was our hope that we would be able to form (S)-equol with high yield and in a timely manner. Successful formation of (S)-equol would then lead to the continued study of this compound against breast, prostate and other forms of cancer, part of which takes place right here at BYU. This would increase the rate at which cancer research of this compound is performed, leading to its use in cancer treatments.
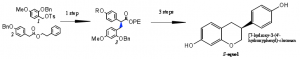
The actual synthesis of the target compound proved difficult. The total route involves eight steps, in other words eight separate reactions (2). The product of each step had to be screened and purified before proceeding with the next step. In the figure below, compounds one and two were reacted together, with the PTC, to form compound three. The PTC was hypothesized to allow formation of only the S enantiomer of compound three, which would eventually lead to the S form of the final product, (S)-equol. This step of the reaction is known in the literature but did not yield compound three when attempted (3). After attempting this reaction numerous times it was determined that compound two, which had been previously synthesized in our lab, was no longer pure and had decayed. More of compound two was made, purified and characterization of it showed data consistent with literature and hypothesized values. Major obstacles were also overcome in the formation of compound one. This compound was difficult to synthesize and involved many variations of reaction conditions and reagents. It was difficult to determine whether or not compound one was successfully synthesized. Nuclear Magnetic Reasonance (NMR) and Mass Spectrometry (MS) are both used to characterize compounds to determine if the reaction was successful. Unfortunately compound one gave a negative MS and the data for the NMR was too similar to the data for the previous step of formation for compound one that we simply had to assume it had been formed and was fit to use for the formation of compound three.
Compound three was never formed in an appreciable enough amount to perform the final steps of the process. The compound (S)-equol was thus never formed nor characterized. The reaction to form compound three was done with and without the PTC present, however both reactions formed little if any of compound three. This could have been due to the lack of pure compound one or possibly from the inability of the PTC to interact with compound two enough to promote the formation of compound three. Thus we cannot at this moment conclude whether or not the use of a PTC would fail or succeed in the formation of (S)-equol.
Although the final steps of this process were not undertaken there is still much work to do on this project. The formation of compound one is a known reaction and further work will eventually provide this compound in its pure form. The formation of compound three is also a known reaction and should work with proper starting materials (4). What we were not able to test was the effect the PTC would have on the stereochemistry of our product. Although this was the sole factor that determined the success of our synthesis we have now joined with another research group on campus headed by Dr. Ess in the Department of Chemistry and Biochemistry to study PTCs and how they interact with other compounds. This is done using density functional theory in the computer program MacroModel. Further work will allow us to calculate the likelihood that various PTCs and compounds will interact with each other (2). This will allow us to look at a wide variety of PTC-molecule interactions and will essentially tell us whether or not the interaction will be favorable enough to promote formation of product. Thus we will now be able to test our hypotheses before using materials in the lab. This will save us time in finding proper PTCs as well as money as we will not have to run as many reactions.
Although this specific project was not successful in that we were able to produce the final product of the reaction it did result in opening the door to exploring many more PTCs as well as other anti-cancer compounds. Our work now focuses on optimizing the PTC-molecular interactions that we hypothesize take place in chemical reactions. We will now be able to expand the number of anti-cancer compounds we can produce because of the versatility offered by computer programs that essentially optimize our reactions for us. Thus this project has succeeded in our overall work in the field of cancer research.
References
- Andrus, M.; Harper K.; Christiansen, M.; Binkley, M. Phase-Transfer Catalyzed Asymmetric Arylacetate Alkylation. Tetrahedron Lett. 2009, 50, 4541-4544.
- Binckley, M. A. Aryl Acetate Phase Transfer Catalysis: Method and Computational Studies. Masters Thesis, Brigham Young University, Provo, UT, 2011.
- Heemstra, Jennifer M.; Kerrigan, Sean A.; Doerge, Daniel R.; Helferich, William G.; Boulanger, William A. Total Synthesis of (S)-equol. Org. Lett. 2006, 8, 5441-5443.
- Versteeg, M.; Bezuidenhoudt, B.; Ferreira, D. The First Synthesis of Isoflavans via α- Alkylation of Phenylacetates. Heterocycles 1993, 36, 1743-1746.
