David Hedges and Dr. Scott Steffensen, Department of Psychology
The purpose of my ORCA research was to better understand the neural circuitry behind addiction. It is well known that the mesocorticolimbic pathway in the brain is involved with addictions. This pathway consists of dopaminergic neurons originating in the ventral tegmental area (VTA) projecting to the nucleus accumbens (NAcc) through the median forebrain bundle (MFB) and the internal capsule (IC). The firing of these dopamine neurons is in part regulated by γ-aminobutyric acid (GABA) neurons in the VTA. It was on this specific GABA population that my research was focused. It was my hypothesis that animals treated with Dermorphin saporin (DS) would not have any active VTA GABA neurons and would not self-administer ethanol when compared to animals injected with unconjugated saporin (blank-saporin: BS).
Though the techniques have significantly improved with time, the practice of creating neural lesions may be the oldest analytical method of neuroscience. In my research, the DS neurotoxin was used. DS specifically targets and kills GABA neurons, effectively creating a cell-specific lesion. Selective lesions can be accomplished by VTA injections of the conjugated DS, which lesions only those neurons expressing μ-ORs. We have demonstrated previously that VTA GABA neurons are sensitive to opioids and contain μ-opioid receptors (1). This toxin was bilaterally microinjected into the VTA of half of the subject male rats, creating a control group and a sham group.
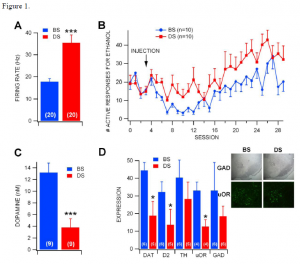
The effect of the neurotoxin on VTA GABA neurons was determined through single-cell electrophysiology recordings. Using stereotactic techniques, VTA GABA cells were isolated and their baseline firing rate was recorded. After the rate was stable, the animal was injected intra-peritoneal with ethanol. These findings are shown in figure 1A, with the firing rate of VTA GABA neurons in DS-injected rats was significantly greater than those in BS-injected rats. This indicates that the DS toxin is able to greatly affect (by nearly 100%!) the behavior of individual neurons.
To determine the effects of DS on reward-seeking behavior rather than simply the activity of a single neuron, rats were trained to self-administer (drink ad libido) ethanol and then injected with DS or BS bilaterally. Following a five-day recovery period, rats were returned to ethanol self-administration. DS rats had more responding for ethanol than BS-injected rats. These results are shown in figure 1B. This shows that that DS affected rats were more likely to engage in addictive behaviors. This shows that the VTA GABA neurons are critical in controlling the drive for this type of behavior.
To further confirm these results, we performed a histological study of the regions of interest. Figure 1D shows these findings. The insets show representative photomicrographs of tissue sections of GAD (a protein specific to GABA neurons) at 4X magnification and μ-opioid receptor (μ-OR) labeling at 20X magnification. The important point of interest is the loss of GAD+ cells in DS-injected rats compared to BS-injected rats, but the dense labeling of the remaining GAD+ cells in DS rats. These findings indicate that DS was indeed targeting the GABA cells, confirming the accuracy of the above findings. Note also the loss of μ-OR+ neurons in DS-injected rats. The overall expression of DAT, D2 and μ-OR were significantly lower in DS-injected rats. This validates the electrophysiological recordings.