Nathan Tulett and Dr. Daniel Maynes, Department of Mechanical Engineering
Background
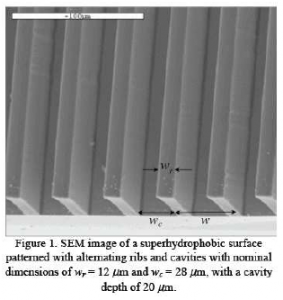
In the last few years a great deal of attention has been given to superhydrophobic surfaces and their impact on drag reduction in fluid flow dynamics. Such surfaces are now employed as self-cleaning substrates and it is expected that their use in the coming years will greatly multiply. In the laboratory, these surfaces often consist of alternating microribs and microcavities that are etched into a silicone substrate using a deep reactive ion etching process (DRIE) as seen in Fig. 1. The surfaces are then coated with a thin hydrophobic coating. As liquid flows past a superhydrophobic surface the fluid alternates between a no-slip region and a virtually shear free region. Trapped vapor in the microcavity leads to the formation of a meniscus which prevents the liquid from wetting. Since the liquid is then capable of wetting only the tops of the microribs the overall contact area and therefore the friction are reduced. Surfaces modified in this manner have produced static contact angles as high as 177° for immobile water droplets and have been shown to reduce the friction in flow channels by up to 11% in turbulent flows and by as great as 60% in laminar flows. While research on these surfaces has been significant, very little attention has been devoted to understanding the impact of superhydrophobic surfaces on heat transfer. The few studies that have appeared have demonstrated that the influence on thermal transport is greater than on the flow dynamics, and the use of superhydrophobic surfaces in novel condensers, heat exchangers, and boiling surfaces could provide significant advantage. In particular, superhydrophobic surfaces are expected to yield a large impact on nucleate boiling, and it is entirely possible that advancement in this area of research will have bigger impact on industry than its fluids applications.
Experimental Procedures
Initial research was focused on establishing an understanding of the impact of superhydrophobic surfaces on nucleate pool boiling by characterizing the boiling curve (originally characterized by Nukiyama for smooth and rough surfaces) for surfaces exhibiting superhydrophobicity. In order to collect the data necessary to establish this curve, a test apparatus was built which holds 3.79 liters (1 gallon) of water. Placed in the apparatus was a vertical 38.1mm (1.5 in) square aluminum surface which was heated using seven 225 Watt cartridge heaters. The aluminum block was embedded in an insulating, closed cell, foam leaving only one face exposed to the water. It was through this surface that heat was transferred and the flux (w/m2) was calculated. The temperature was measured using thermocouples and de-ionized water was preheated to 90°C to reduce the required flux for boiling and reduce the gas content in the water. Preliminary experiments with just the aluminum block provided data that was consistent with well-established heat transfer boiling curves. The silicon surfaces were placed on the aluminum heating block using a thermal paste and a calibration curve was established for the thermal resistance using an IR camera to determine the actual surface temperature. However, underwater tests showed the calibration curve to be inaccurate and the thermal resistance became greater than that of air. This resulted in the aluminum block reaching temperatures around 500°C which was damaging to the apparatus and in general unsafe.
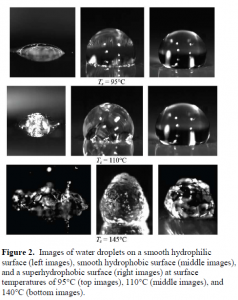
The research focus was then modified to establishing an understanding how superhydrophobic surfaces affect the boiling of water droplets. With the use of a high speed camera, images were taken to demonstrate how these surfaces shifted the boiling curve for three temperatures. The test set-up used an aluminum block imbedded in an insulating, closed cell, foam. This block was heated using a heating patch and the surface temperature was determined using a thermocouple. An IR camera was used to compare surface temperatures with the thermocouple and a calibration curve was again established. Once the surface was heated and the temperature was at steady state, a pure water droplet was released from a syringe and the high-speed camera was initiated. Smooth silicon, smooth hydrophobic and superhydrophobic surfaces were tested at temperatures of 95°C, 110°C, and 140°C. Video was recorded at 9000 frames per second and images were selected from these recordings.
Results
The three surfaces evaluated have distinctly different contact angles which can be seen in the images taken at 95°C in Fig. 2. Since 95°C is below the saturation point there are no disruptions to the surface of the droplet and there are no signs of boiling for any of the surfaces. However, it is understood that, although not visible, convection currents are undoubtedly present within the droplets. As the surface temperature is increased to 110°C we can see increased activity within the droplets. The silicon surface begins to boil and
vapor bubbles that have formed within the droplet escape and disrupt the surface. The droplet on the hydrophobic surface shows visible surface distortion due to increased convective currents, but does not exhibit any boiling phenomena. The superhydrophobic surface shows no visible distortions or boiling. At temperatures of 140°C and higher the silicon surface will boil violently. The hydrophobic surface droplets have intense convective currents that rise quickly then cool which distorts not only the surface of the droplet but also the shape. This up and down motion occurs rapidly and the droplet is quickly driven from the surface. The superhydrophobic surface droplets still show no signs of boiling but do see significant surface deformation. The surface motion of the droplet is rapid, however, unlike the hydrophobic surface the droplet remains in the original dropped location. In addition, Leidenfrost behaviors are seen at temperatures above 160°C but are not associated with boiling. Understanding why these phenomena occur will be the focus of future research.