Trevor Slade and Dr. Morris Argyle, Department of Chemical Engineering
Introduction
The water-gas shift reaction is the reaction where carbon monoxide and water react to form carbon dioxide and hydrogen as shown in equation 1.
The reaction is commonly used alongside steam reforming of methane to produce high purity hydrogen which is used to synthesize ammonia on an industrial scale. The reaction is exothermic and follows Le Chatlier’s principle because the yield decreases with a temperature. The process is typically split into two stages, the first called the high-temperature shift which operates at 350 centigrade and the second called the low-temperature shift which operates near 200 centigrade. In industry, iron based catalysts are used to catalyze high temperature shift reactions and copper on zinc and aluminum oxide catalysts are used to catalyze low temperature shift reactions.
The purpose of this project was to determine the effect of lanthanum on the activity and stability of iron based catalysts in a high temperature water gas shift reaction. Longevity is important for catalysts because replacing them requires process disruption. Activity is important because it increases the conversion rate per unit area which decreases the size of the reactor.
Catalyst Synthesis
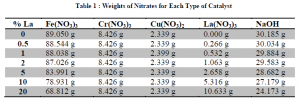
The co-precipitation method was used to prepare each catalyst. The chemicals used for the catalyst formation are shown in table 1.
Each of the compounds were weighed out and dissolved in distilled water. After the compounds were dissolved, sodium hydroxide was added until the PH of the solution stabilized at 11. The slurry was vacuum dried and then oven dried at 60 degrees centigrade for 25 hours. After the catalyst was dry, it was calcined by heating it to 300 centigrade for 3 hours. Lastly, the catalyst was sifted and the particles between 125 and 250 micrometers in diameter were used.
Kinetic Experiment
0.1 grams of catalyst was placed in a quartz reactor that was one eighth of an inch in diameter. The catalyst was first reduced by flowing argon, hydrogen and water into a reactor at 400 centigrade for two hours. Then, the reaction was started by flowing water and carbon monoxide using a 3:1 molar ratio. The reaction was allowed to equilibrate at 400 centigrade, after which the catalyst’s performance was tested at 350, 375, 400 and 425 centigrade. This testing was done for each type of catalyst (differentiated by its weight percentage of lanthanum). The reaction rate was measured by determining the conversion rate using gas chromatography while the surface area and porosity of the catalyst were determined using the BET method (Brunauer, Emmet and Teller).
Results and Conclusion
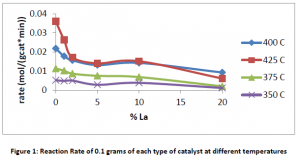
Although lanthanum is used to increase the longevity and activity of other types of catalysts, data from the experiments performed in our lab indicate that lanthanum does not improve the activity iron based water-gas shift catalyst; on the contrary it decreases performance. Figure 1 shows the reaction rate of the reactor for different catalysts at different temperatures.
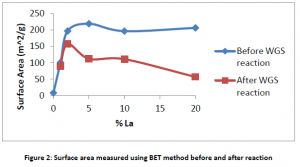
The impact of lanthanum on the longevity of the catalyst is capture by measuring the surface area, pore volume and pore size before and after 12 days of reactions take place. The surface area is the best indicator of a catalyst’s physical formation, and a decrease in surface area indicates that the catalyst is deactivating. As shown in figure 2, the surface area remains constant for the reacting period when using less than 2% lanthanum. After 2%, the surface area drops substantially suggesting that the catalyst is less stable when using a high weight percentage of lanthanum.
The results suggest that lanthanum is not a good additive to iron based water shift catalysts, especially above 2% by weight. It is recommended that future research be done between 0 and 1.5% lanthanum to make sure that the data follows the trend shown in figures one and two.