Joseph Mosley and Dr. Jaron Hansen, Department of Chemistry and Biochemistry
The effects of water vapor on the kinetics and product branching ratio of the reaction of organic peroxy radicals (RO2) with nitrous oxide are under investigation. Hyrdrocarbon combustion produces organic peroxy radicals which then react with NO to form NO2 or organic nitrates (RONO2). This reaction which branches is shown below:
Reaction 1 has two possible channels, the oxidation of the NO to form NO2 (1a) and the association reaction to form an organic nitrate (1b). The formation of the NO2 is readily photolyzed (2) in the daytime to form tropospheric ozone (3). In the troposphere, ozone is a pollutant which has proven to aggravate cardiovascular systems.1
In the absence of water vapor, the above reactions proceed at a certain branching ratio (1a/1b), but the addition of water vapor perturbs the system to produce more organic nitrates. The formation of organic nitrates is important because they have a significantly greater stability than radicals and consequently their formation serves to produce a stable species that can be transported to otherwise pristine areas of the atmosphere where they consequently break down to produce ozone formation precursors. In order to properly model the atmosphere and the influence of various pollution sources on overall air quality it is important to understand the product branching ratio for reaction of organic peroxy radicals with NO. Quantifying the amount of organic nitrates formed is challenging but by measuring the amount of NO2 produced in the presence and absence of water, the change in the product branching ratio can be determined.
Laser induced fluorescence (LIF) is used to detect NO2. Previous work by the Hansen lab measured NO2 fluorescence as a function of pressure below 50 torr. This experiment has a similar system, but requires pressures of NO2 above 50 torr. My research was to make calibration curves for NO2 with an LIF system for pressures between 50 and 200 torr.
Methods
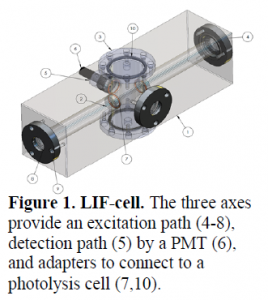
A doubled-frequency Nd:YAG (532 nm) pulsed laser and photon counter were used to facilitate fast rep rates (~8.5 KHz) and short (5 μs) gated collection windows. As seen in Figure 1, the cell was set up and the laser aligned with a half-wave plate to properly orient the polarization of the laser through the cell. The cell was connected to vacuum with a MKS Baratron pressure transducer and to a manifold that delivers known concentrations of NO2. The photomultiplier tube (PMT) is connected to the photon counter and placed orthogonally to the beam excitation at the angle of least Rayleigh scattering to detect fluorescence.
After setting up the instrument, preliminary results were taken revealing a lot of background noise. A 532 nm notch filter along with three 560 nm high pass filters were placed in front of the PMT to reduce laser scattering reaching the PMT. Adjusting the PMT and the delay on the photon counter was also necessary to get good results. A LabView program was made with the help of Taylor Cline and consisted of a program that communicated with the photon counter to record the number of photons detected by the PMT every two seconds.
Results
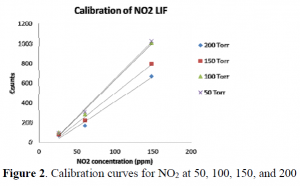
Initially the data showed that the signal-to-noise ratio was too low and also that quenching at such high pressures was too excessive. After adding necessary filters, good data was collected. Results from the LIF portion of this experiment are displayed in calibration curves. The data for each calibration curve was taken on the same day to make sure that the instruments were working uniformly. An example of a calibration curve is shown left in Figure 2 showing LIF of NO2 at 50, 100, 150, and 200 torr.
Discussion and Conclusions
Results show that a calibration curve at higher pressures (50-200 torr) is possible. The unsuspecting problem was that the signal to noise ratio not being high enough. Higher pressures cause quenching which is allowed because the excited state of NO2 has a long lifetime. To reduce scattering and background noise, it was imperative to use filters. This really helped to lower the background noise. Laser alignment was also important in reducing the number of background counts. Extensive static experiments were performed to gain a full understanding of the effects of pressure and the concentration of quenchers (N2, O2) on the detection limits of NO2 using LIF.
In conclusion, this experiment shows that NO2 concentration can be discovered using LIF even with higher pressures. Quenching lowers the signal to a large degree, but the calibration curve still shows a linear relationship between the number of counts and the concentration of NO2. Future work will be done to understand how water vapor quenches LIF of NO2. This will help in the larger experiment to know the correct branching ratio based off the correct concentration of NO2.
References
- Jerrett, M.; Burnett, R.T.; Pope, C.A.; Ito, K.; Thurston, G.; Krewski, D.; Shi,Y.; Calle, E.; Thun,
M. Long-Term Ozone Exposure and Mortality N.Engl.J.Med., 2009, 360, 11, 1085-1095.