Scott L. Thomson and Dr. Dan Maynes, Mechanical Engineering
Developing a method of measuring fluid temperature using a laser-based system was the goal of this research project. The following paragraphs summarize the research procedure, results, and plans for future development.
The temperature measurement technique is based on molecular tagging velocimetry (MTV), an established, non-intrusive method of measuring fluid velocity using a laser. MTV works by introducing into water the photoluminescent chemical 1-BrNp×Gb-CD×ROH ternary complex (hereafter referred to as Gb-CD), directing an ultraviolet laser beam through the solution, and imaging the phosphorescence resulting from the return of the chemical’s molecules to their unexcited states following excitation by the laser. An important characteristic of this process is that the intensity of the emitted light is inversely related to its temperature. Our goal has been to quantify this temperature-intensity relationship so that ultimately, simultaneous measurements of temperature and velocity using MTV will be possible.
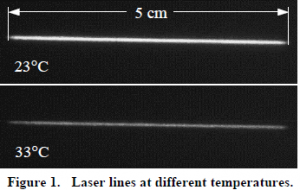
A small, transparent apparatus was filled with a solution of Gb-CD. A thermocouple was placed in one part of the fluid and a resistance heater in another part. The fluid was heated to a predetermined temperature and a beam derived from an ultraviolet excimer laser was directed through the fluid. A CCD detector then captured a series of consecutive images of the resulting phosphorescence and transferred these images to a computer for analysis. The fluid was heated to another temperature, more images recorded, and so forth until the intensity of the beam was no longer observable. Figure 1 shows two images of the beam acquired by the CCD at two different fluid temperatures.
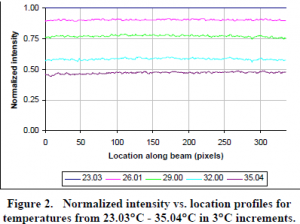
A program (written in C) was then developed to quantify the beam images. This data was normalized to the beam of maximum intensity (which corresponded to the minimum fluid temperature). This resulted in being able to relate the relative phosphorescence intensity of the beam to the temperature of the fluid. Fig. 2 shows the relative intensity of the beams at different fluid temperatures from one experiment.
From this figure it can be qualitatively observed that there is a relationship between temperature and phosphorescence intensity. The change in intensity with temperature can also be quantified. For this data set for example, a change in temperature of 12°C resulted in an intensity decrease of approximately 50% from the initial intensity.
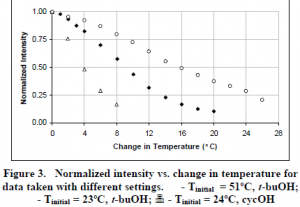
This procedure was repeated for a number of different settings, temperatures, and solution constituents. Fig. 3 shows the averaged intensity of the beam lines versus change in temperature for images taken at different initial fluid temperatures (Tinitial) and alcohols used in solution (t-buOH vs. cycOH).
It can be seen that with t-buOH, the intensity decreases much more rapidly at the higher initial temperature ( ) than at the lower initial temperature ( ). Thus at higher temperatures, better resolution is possible while at lower temperatures, greater range is possible. Also, using cycOH resulted in a much greater range (6).
Much progress has been made towards the goal of extending MTV to thermometry and further development is a significant focus of the author’s graduate research at BYU.