Rebecca Stevens and Dr. Juliana Boerio-Goates, Chemistry and Biochemistry
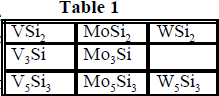
Vanadium, molybdenum, and tungsten silicides are inorganic materials that have many practical applications at high temperatures and high pressures1. Eight compounds which are of particular interest are found in Table 1. Accurate thermodynamic data aids in the synthesis of high purity, single phase materials. For example, knowledge of the Gibbs free energies as a function of temperature and pressure allows one to predict relative stabilities of the binary compounds. Calculation of this thermodynamic property requires the enthalpy of formation of these materials as well as their entropies and heat capacities. The enthalpies have been reported for many of these compounds at room temperature.2,3 However, the low temperature heat capacity data needed to obtain the free energies are not generally available. In addition the high temperature heat capacity data used to extend the free energies to high temperatures needed for synthetic applications show considerable disagreement.1
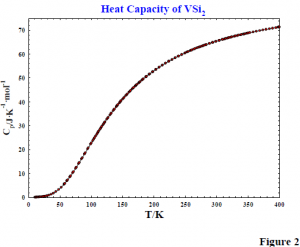
The heat capacity of VSi2 was measured with an adiabatic cryostat from 15 to 400K. Sample characterization included Scanning Electron Microscopy and chemical analysis by a commercial laboratory. Results indicate material of high chemical and phase purity.
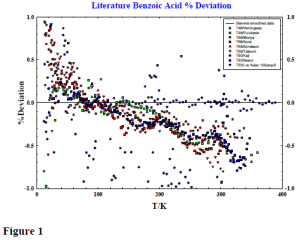
In order to check the accuracy of our apparatus, the heat capacity of benzoic acid, a standard reference material, was also measured. It is customary to perform this check whenever major instrumental changes are made to the system. Historically, in the best laboratories measurements made on large samples have agreed to within 0.5% to 1% at temperatures less than 30K, 0.2% to 0.3% between 30 to 50K, and better than 0.1% from 50 to 350K. Figure 1 shows the deviation of reported heat capacity values from our results. In every temperature range, our deviations are systematic and larger than we expected.
The systematic nature of these deviations suggest that there is an energy loss not accounted for in the measurements. In December 1998 new shield temperature controllers were installed. It was found that cooling wires were needed to improve thermal conductivity between the shield and the calorimeter in certain temperature regions. We suspected that these wires served to carry away heat.
Measurements were made on an empty calorimeter both with and without wires. The results showed that the wires were not the source of the energy loss but improved the response time of the main shield. Calculations showed that the energy loss increased strongly with temperature, suggesting that radiative loss rather than conduction was the source. A black silk string attached to the thermometer-heater assembly is now the most likely culprit. We are now making measurements without the string to verify this assumption. Preliminary results are encouraging.
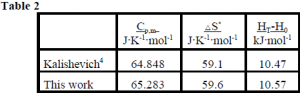
We have used one set of results to calculate thermodynamic functions for VSi2. The heat capacity of VSi2 is shown in figure 2. Thermodynamic functions have also been calculated at 298.15K. They are given in table 2.
References
- Schlesinger, Mark E. Chem. Rev. 1990, 90, 607-628 and references there in.
- Tomaszkiewicz, I.; Hope, G.A.; Beck, Charles M., II; O’Hare, P. A. G. J. Chem. Thermodyn. 1997, 29, 87-98.
- O’Hare, P.A.G., Watling, K., Hope, G.A., J. Chem. Thermodyn. in press (1999).
- Kalischevich, G. I.; Gel’d, P.V.; Krentsis, R. P. Zh. Fiz. Khim. 1968, 42, 1288-1289.