Emily Parker and Dr. Merritt B. Andrus, Chemistry and Biochemistry
Chemotherapy is one of the primary treatments for cancer today. A patient’s life may depend on its efficacy, so when he or she develops multi-drug resistance (MDR) the results can be devastating. MDR, a clinical condition manifested by the failure of chemotherapy, is primarily due to the expression of P-glycoprotein (Pgp), a membrane-bound small molecule transport protein which is found normally in some cells but is greatly over expressed in drug resistant cancer cells1. It is believed that after chemotherapy drugs diffuse into the cell, Pgp effluxes them out using energy from ATP hydrolysis, thus imparting drug resistance.
We are interested in studying the three-dimensional structure and mechanism of Pgp, which includes discovering the exact locations of its binding sites. The basic structure of Pgp is a pore composed of twelve transmembrane helices. Helices TM6 and TM12 are thought to contain the protein’s binding sites, but their locations are unknown and a definitive model of Pgp’s three dimensional structure has been hard to obtain because direct structural analysis of membrane bound proteins is difficult. Determining the structure of Pgp, then, must be done by indirect experimental techniques 2.
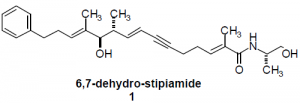
6,7-dehydro-stipiamide (6,7-DHS), synthesized by Andrus et al3, inhibits Pgp and thus reverses MDR. It is thought to bind the same sites in Pgp as chemotherapy drugs, and can be used to probe the structure and mechanism of Pgp. I proposed to link two of these compounds together with a polyethylene glycol linker to make a bifunctional homodimer. By measuring the MDR reversal potency in drug resistant cells treated with these dimers, the distance between TM6 and TM12 can be studied; the dimer with highest potency will indicate that the particular tether length used is the approximate distance between the two helices. With this technique we hope to establish whether the helices are adjacent or opposite each other in the pore and provide, for the first time, insight into the binding characteristics and mechanism of transport of this protein.
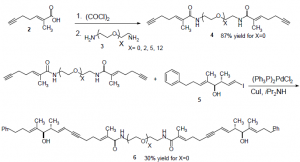
I successfully synthesized homodimers (compound 6) of tether lengths X=0, 2, and 5 using the scheme below. Linkers (3) of lengths X=0 and 2 were commercially available but I had to synthesize the X=5 diamine from hexa(ethylene glycol) using a three step procedure, with a 61% overall yield.
I planned to synthesize homodimers of lengths X=8 and X=12, but this was more difficult because polyethylene glycols of these lengths are not commercially available. I spent a great deal of time and effort attempting to synthesize longer linkers using a procedure for the synthesis of oligo(ethylene glycols)4 but was unfortunately unable to get the reactions to proceed with high enough yield to proceed with synthesizing the homodimers. I have since obtained a different scheme for synthesizing longer polyethylene glycols and am currently experimenting with it.
Homodimers of lengths X=0, 2, and 5 were sent to our collaborators at NIH and Purdue University for cell assays. At this point we have no results for tests on their MDR reversal potency, but assays were done measuring their effect on ATP hydrolysis by Pgp and their binding affinity to Pgp. Monomeric MDR reversal compounds such as 6,7- DHS inhibit ATP hydrolysis, but the X=0 homodimer does not, and the X=2 homodimer does to a lesser degree. The homodimers bind Pgp slightly less strongly than the monomers, but there is no apparent correlation between ATP hydrolysis and binding affinity; it appears that the homodimers are binding through a different mechanism than the monomers. Pgp operates by a complex mechanism and we still do not understand how these compounds inhibit ATPase activity or reverse drug resistance. Our research has opened many new questions for future exploration.
References
- Ferry, D. R.; Traunecker, G.; Kerr, D. J. Eur. J. Cancer 1996, 32A, 912-920.
- Higgins, C. H.; Callaghan, R.; Linton, K. J. Cancer Biology 1997, 135-142.
- Andrus, M. B.; Lepore, S. D.; Turner, T. M. J. Am. Chem. Soc. 1997, 12159-12169.
- Jenneskens, L. W.; Keegstra, E. M. D.; Zwikker, J. W.; Roest M.R. J. Org. Chem. 1992, 6678-6680.