Jacob Hedelius and Dr. David Dearden, Department of Chemistry and Biochemistry
Mathematical modeling is both common and increasingly expected in scientific research, to both predict and to explain results. Models are used in many disciplines, from describing cellular process in biology, weather patterns in planetary science, to galaxies in physics. As part of my studies with the Office of Research and Creative Activities (ORCA), I created a simple model for reactions of the molecule decamethylcucurbit[5]uril (mc5) in the gas phase.
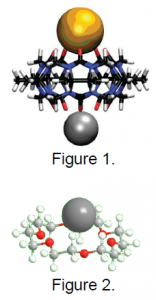
Mc5 is pumpkin-shaped molecule, and has been the subject of several studies in the Dearden group.1–3 This molecule is interesting because it can bind to other molecules or to positively charged atoms (cations) on either end, creating a very small cage that can trap small molecules such as oxygen, nitrogen, and methanol.2 This situation is illustrated in Figure 1 from reference 1. Here mc5 is bound to a charged cesium atom (gold) and a charged lithium atom (silver), with an empty cage. Studies on this molecule have potential application in storing molecules and atoms for future use, or waste removal.
Mc5 is not the only molecule that can bind to cations. Crown ethers, such as 18-crown-6, bind more strongly to some cations than mc5. Figure 2 shows 18-crown-6 bound to a charged lithium atom. Because crown ethers bind more strongly to cations, when conditions are right, a crown ether will steal a cation from an mc5 with cations.
In my model I made three conditions necessary for the exchange of a cation from mc5 to a crown ether to occur. First I required that the mc5 complex and the crown ether needed to collide. How often the molecules collided was based on temperature (related to speed), pressure (related to how many are present in a fixed volume), and size. Next the reaction only occurs when both molecules are oriented right – the top of the crown ether has to contact the cation it is stealing. Finally I required that there was enough energy for the reaction to occur based off of temperature.
Most models require some assumptions and simplifications. In this model I made many assumptions, such as the location of a guest trapped in the cage, shapes and sizes of molecules and cations, and the energy available and required to react. Assumptions and the model in general gave insight into the reaction and reaction conditions.
For the most part, the reaction trends matched those observed in experiments. So far experimental studies have been conducted when there is no crown ether present, and instead an unreactive atom such as argon hits the mc5 complex hard enough or collides with enough energy that a cation falls off.1 Of the 7 different cation combinations in the model, 6 followed the expected trend. The seventh likely didn’t follow the trend because in my model I may have assumed the cations to be too large.
Though some studies on the crown ether/mc5 reaction have been performed,2 there is still more to study. I attempted to study this reaction experimentally in a mass spectrometer. Techniques to get mc5 complexes into the gas phase in the mass spectrometer are routine and I was able to detect the mc5 with various metal combinations. However, crown ethers are more difficult to get into the gas phase. Though I tried several methods to vaporize the crown ether such as heating it up, and placing it under low pressure, I never detected it. I expect that a simple addition to the instrument – a solids probe – will be a means that will easily get the crown ether into the gas phase.
I was able to present this model at the College of Physical and Mathematical Science (CPMS) 26th annual Student Research Conference.
I am very grateful to Brigham Young University, ORCA, and Professor Dearden for allowing me to learn about, and teaching me principles of sound scientific research. By being a part of undergraduate research in the Dearden group I gained understanding of performing original research with a group, reviewing and researching current scientific literature, understanding instrumentation, writing proposals, papers, and presenting scientific results. By attending group meetings I learned about problem solving in modern scientific research. I largely attribute being accepted into several top graduate programs in chemistry to my undergraduate research experience at BYU.
Now I am in my first year of graduate studies in chemistry in the Ph.D. program at the California Institute of Technology. Though my research in graduate school may have little similarities to this project, skills I gained from undergraduate research in logical reasoning, and working as a scientific team will carry with me throughout my entire life.
References
- Mortensen, D.N.; Dearden, D.V. Influence of charge repulsion on binding strengths: experimental and computational characterization of mixed alkali metal complexes of decamethylcucurbit[5]uril in the gas phase. Chem. Commun. 2011, 47, 6081–6083.
- Kellersberger, K.; Anderson, J.D.; Ward, S.M.; Krakowiak, K.E.; Dearden, D.V. Encapsulation of N2, O2, Methanol, or Acetonitrile by Decamethylcucurbit[5]uril(NH4+)2 Complexes in the gas Phase: Influence of the Guest on “Lid” Tightness. J. Am. Chem. Soc. 2001, 123, 11316–11317.
- Mortensen, D.N.; Dearden, D.V.; Zhang, H.Z.; Allred, M. Inert but not inactive: Alkali cation complexes of decamethylcucurbit[5]uril with a Xe atom trapped in the cage. Abstracts of Papers of the American Chemical Society, 241st National Meeting and Exposition of the American-Chemical-Society (ACS), Anaheim, CA, Mar 27–31, 2011.