Andrew Broadbent and Dr. Daniel Simmons, Department of Chemistry and Biochemistry
The laboratory of Dr. Daniel L. Simmons, my mentor, studies the cyclooxygenase (COX) enzyme. Dr. Simmons is one of the world’s foremost authorities on COX, he having discovered one of its two known types, COX-2. The COX enzyme, which exists in many organisms, including humans, is responsible for producing molecules that trigger inflammation. For this reason, understanding COX is important: for example, aspirin works by deactivating the enzyme, and COX could play a role in the inflammation linked to diseases like type-II diabetes and cancer. Thus, greater knowledge of its workings could aid those suffering from a variety of diseases.
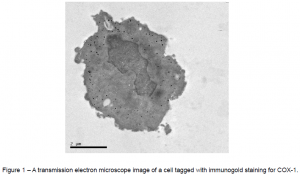
Over the past few years, the Simmons team has discovered new proteins associated with this enzyme, and we are currently studying their location and function. My part of the research is to take pictures of where these proteins reside in living cells. When I applied for an ORCA grant in December of 2011, my goal was to obtain successful pictures by the end of March 2012. Because cells and their parts are so small and we wanted a high level of detail in the pictures, I needed a transmission electron microscope (TEM). To see where the enzyme molecules are located, I tagged them with something that would be visible under the electron microscope—microscopic gold beads. This technique is known as immunogold staining. The gold beads are dense enough that electrons do not pass through them, so under the electron microscope, they show up as black dots against the grey background of the cell. This procedure has been used successfully many times, but no one at BYU has yet been able to do it reproducibly. Nevertheless, with the guidance of on-campus electron microscopy technicians and a protocol from a similar, already-published study, I set out to learn it. After training in electron microscope operation and ordering the special chemicals needed for the procedure, I spent the next several weeks attempting the procedure.
My first experiment was a near-complete failure, but I learned from my mistakes, and the next tries saw improvement. By the end of March, I had succeeded in obtaining images of successfully tagged COX-1 enzyme—not the new proteins I eventually need to tag, but a predictable, well-understood test molecule. Three weeks later, I repeated those results, proving that I have a reproducible method. This alone was groundbreaking progress not only for me, but also for microscopy at BYU in general. Figure 1 at the end of this text is one of the images I took using one of BYU’s transmission electron microscopes. Despite the successful tagging, however, the delicate structures of the cells were compromised. This is the most difficult part of the goldstaining technique: what preserves structure tends to lessen tagging, and what helps tagging tends to damage structure. This meant that I had to strike a balance between the two, since I needed to preserve both structure and tagging.
Because much of the damage to tagging came from using harsh chemicals to preserve the structure, I decided to try freezing the cell instead. That proved unfruitful too as ice crystals formed and severely damaged the cell structure. I was told that even liquid nitrogen, the best means of freezing available on campus, would not freeze the cells quickly enough to avoid ice crystal formation, and so I abandoned the freezing technique and turned back to chemical treatments. The experiments that followed during the spring and summer saw some minor improvements, but the quality of tagging and structure preservation were still nowhere near where they needed to be. It seemed as if I had come to an impasse.
During the fall of 2012, I did not work on the project except to make arrangements to use the University of Utah’s high-pressure freezing equipment for the freezing technique which I had given up on earlier. Come the start of this year, I returned to active duty in the lab, and I realized that the protocol I had been following included a successful freezing procedure which did not require high-pressure equipment. Instead, it relied upon light chemical preservation followed by infusion with sucrose, which would depress the freezing point of the intracellular fluid. The cells could then be frozen in liquid nitrogen—cold enough to preserve the cellular structure, yet not cold enough to freeze the sucrose-infused intracellular fluid. So, rather than travel somewhere else, I decided to stay at BYU to pursue the freezing technique as detailed in the protocol. I also obtained a couple chemicals listed in the protocol which I had considered minor and had left out in my experiments. Looking back, I see that I should have used them. Right now I am beginning an experiment which will use the improved freezing technique as well as the missing chemicals, and I am eager for its results.
Once I am able to repeatedly obtain high-quality election microscope images, the next task will be to obtain as many of them as possible: first for the new proteins we are studying, and also for COX in general. Dr. Simmons told me that the electron microscopy of COX is lacking in scientific literature, and that I could make a significant contribution by adding to it. I am grateful for the help and encouragement of Brigham Young University and the ORCA program which have allowed me opportunities to experience world-class research.