K.R. Pitts and G.D. Watt, Department of Chemistry and Biochemistry
Introduction
I ron is an essential mineral nutrient required by all living systems. How Iron Is taken up, stored, and utilized by living systems is relevant to the understanding of life In general, and the relationship Iron metabolism has with human health. Azotobacter Vinelandii bacterial ferritin (AVBF) represents the bacterial protein molecule Involved In Iron storage and regulation. The 24 protein subunits and 12 heme groups that comprise A VBF arrange themselves structurally to form a hollow sphere in which AVBF stores Iron. The arrangement of protein subunits create channels roughly 50 nm in diameter that traverse the protein shell. These channels facilitate Iron transport and storage, a process governed by oxidation-reduction events on the protein’s surface. Spectrophotometric methods to monitor the iron release process, as well as reduction events at the protein surface, have been developed In our lab In order to characterize and understand the mechanism by which iron is released from AVBF.
Experimental
AVBF containing 12 heme groups and 5 core Iron atoms was prepared [Treffry and Harrison, 1978] from holoAVBF containing 1575 iron atoms by dialysis against thioglycolic acid. The heme content was determined by optical spectroscopy [Watt eta!., 1993]. Total iron content was determined by inductively coupled plasma (ICP) spectroscopy and total reduction capacity (heme plus core iron) was determined by microcoulometry [Watt eta!., 1986]. This AVBF sample was loaded anaerobically into an Applied Photophysics DX. 17MV Sequential Stopped-Flow Spectrophotometer and the rate of reduction with dithionite was measured at 25’C and pH 7.5. The formation of reduced heme was monitored at 557.5 nm, the wavelength of maximum absorption of the reduced heme aband. Identical measurements were taken using holoA VBF containing 480 iron atoms to assess the effects a larger core would have on the rate of heme reduction. In both of these cases the core iron was reduced by dithionite but was retained within the A VBF interior. A sample of holoA VBF containing 480 iron atoms used In the aforementioned experiment was prereduced anaerobically with dithlonite In the absence of bipyridine (bipy) to form the reduced iron core. The sample was then moved anaerobically to the stoppedflow apparatus and the rate of iron removal from the reduced core by bipy was measured.
Results
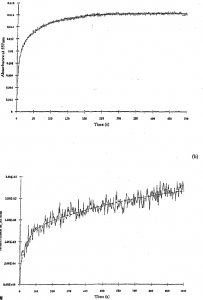
Figure Ia represents a stopped-flow kinetic trace for the reduction of AVBF containing 12 heme groups and 5 core iron atoms at 557.5 nm. The reductant used was dithionite. This curve is a composite of two first order heme reduction reactions where an initial rapid reaction (rate constant k1 = 0.223 sec·’) is seen, followed by a second slower reaction (rate constant k2 = 0.0137 sec-1). Other data not presented in this article were consistent with the reported results above, namely that two first order rates differing by a factor of ten were observed. The results in figure Ia can by interpreted by the following kinetic scheme in which the Initial rapid step Is reduction of the Fe … into Fe” in the heme present In AVBF  where II and III correspond to heme group iron atoms and 2+ and 3+ correspond to core iron atoms.
where II and III correspond to heme group iron atoms and 2+ and 3+ correspond to core iron atoms.
Analysis of the fractional distribution of data points in Figure I a shows that only 78% of the heme groups in AVBF are reduced because reduced heme is then reoxidized by electron transfer to the 5 iron atoms in the core in a slower step. Heme groups oscillate between reduced and oxidized states until all the core iron is reduced at which point the entirety of A VBF heme groups are completely reduced. Figure I b shows a similar AVBF kinetic trace on a sample containing 480 core iron atoms. Although their rate constants are slower than AVBF samples with small cores, large core samples also exhibited two first order rates differing by a factor of ten. With a larger core size, the level of heme reduction is smaller and the length of time required to attain complete reduction is longer because a larger number of core iron atoms must be reduced via heme. The initial level of heme reduction in AVBF with larger cores is smaller and kept at a smaller, near steady state level of reduction until nearly all core iron atoms are reduced. Our results suggest that the conversion of core Fe3+ into core Fe2+ is the rate limiting step in iron release from AVBF.
The author wishes to acknowledge research support from the National Institutes of Health, the Undergraduate Research Program of the College of Physical and Mathematical Sciences and the Office of Research and Creative Activities at Brigham Young University.
References
- [Treffry and Harrison, 1978[ Treffry, A. and Harrison, P. (1978). Biochem. ]., 313.
- [Watt et al., 19861 Watt, G.D., Frankel, R. B., Papaefthymiou, G. C., Spartalian, K., and Stiefel, E. I. (I 986). Biochemistry, 4330.
- [Watt et a!., I 993] Watt, G.D., McDonald, J.W .. Chiu, C.H., and Reddy, K.R.N. (1993). ]. Inorg. Blochem., 745.