Terry S. Elton and Dr. James W. Ogilvie Jr., Biochemistry and Chemistry
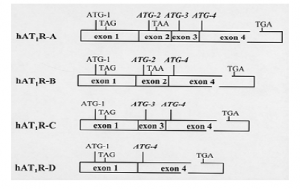
Hypertension (i.e., high blood pressure) is a significant health problem affecting more than 15% of the population contributing to the increased incidence of heart failure, kidney failure and stroke. The renin-angiotensin system (RAS) plays a pivotal role in salt and water homeostasis and the maintenance of vascular tone. This balance is achieved through the actions of the hormone, angiotensin II (Ang II), the biologically active component of the RAS, with its membrane-bound receptors. These receptors, designated as type 1 and type 2 (AT1R and AT2R), bind Ang II with equal affinity. The AT1R, however, which is localized in numerous tissues and cell types, is responsible for mediating most, if not all, of the well known physiological effects of Ang II. Our laboratory has shown that the human AT1R (hAT1R) gene contains at least four exons and is encoded by four distinct alternatively spliced mRNA transcripts (1) (Figure 1). Sequence analysis suggested that the hAT1R-A and -C transcripts may encode a novel hAT1R that has a 32 amino acid extension at the amino terminus given that exon 3 harbors an ATG translation initiation start site that is in frame with the known ATG start site located in exon 4. Therefore, the specific aim of this proposal was to investigate whether hAT1R mRNA transcripts $A# and $C# generate a novel hAT1R and to determine whether these receptors are functionally distinct.
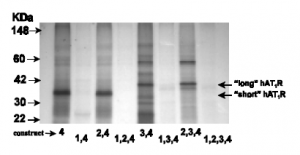
In order to investigate the hypothesis that transcripts containing exon 3 encode a novel hAT1R, we first demonstrated that mRNAs containing exon 3 synthesized the receptor in vitro. Utilizing an in vitro transcription/translation system, the hAT1R constructs harboring the appropriate combination of exons were tested for production of the novel protein. This novel hAT1R-long (i.e., protein made from translation initiation at the ATG in exon 3) isoform encoded a protein ~4,000 daltons larger than the hAT1R-short (i.e., protein made from translation initiation at the ATG in exon 4) isoform (Figure 2). These proteins were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
To demonstrate the authenticity of the novel hAT1R isoform, the appropriate hAT1R constructs were transfected into established cell lines and tested for the ability of the novel isoform to bind angiotensin II and signal transduce appropriately (i.e., inositol phosphate production and intracellular calcium mobilization). Radioreceptor binding assay results proved that the $long# isoform indeed bound angiotensin II (including other ligands) with the same affinity as the $short# isoform. Despite being pharmacologically similar, the two isoforms did however demonstrate functional differences.
To track these differences experiments were performed to show that each transfected cell line expressed approximately equal number of receptors (data not shown). The data from figure 3 suggests that a greater angiotensin II-induced calcium (Ca2+) response is observed in the “long” hAT1R transfected cell line when compared with the “short” hAT1R transfected cells. Similar results occur with angiotensin II-induced inositol phosphate production (Figure 4). These experiments confirm that the $long# isoform is indeed a hAT1R and that it elicits a more pronounced response to angiotensin II which cannot be attributed to a greater number of receptors.
Our laboratory is continuing to make direct comparisons between these two isoforms. We are still working on developing functional antibodies that cross-react with only the novel hAT1R-long isoform in order to investigate which human tissues express the novel receptor in vivo. This research focused primarily on the hypothesized presence of the novel hAT1R-long isoform and has led to a better understanding of the role that the 51-untranslated exons play in receptor expression. Having demonstrated that different mRNA transcripts lead to different receptor functions, it is our hope that specific mRNA transcripts may be linked to the pathogenesis of hypertension and its many accompanying health problems.
Reference
- Su, B., Martin, M.M., Beason, K.B., Miller, P.J. and Elton, T.S. The genomic organization and functional analysis of the promoter for the human angiotensin II type 1 receptor. Biochem Biophys Res Commun 204:1039-1046, 1994.