Sarah Morgan, Chemistry
For my creative work and research scholarship project I proposed the synthesis of four polymers for use as stationary phases in gas chromatography. Each polymer would contain a pendant group with a halogen-substituted benzene ring, making use of the four halogen atoms-fluorine, chlorine, bromine, and iodine. Since these atoms lie along a spectrum of polarity vs. polarizability, I hypothesized that testing the selectivity of the polymers as GC phases would increase our understanding of the roles of these closely related properties in chromatographic separations. I have chosen to submit selected nuclear magnetic resonance spectra as evidence of the success of the synthesis (see Table of NMR Spectra, page 5). Unfortunately, chromatographic testing is still being performed and these data are not yet available.
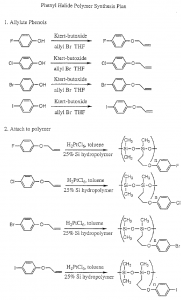
The synthesis plan is outlined in Scheme 1 (page 3). The first step of the synthesis was accomplished as planned. The para-halide phenols were obtained from sources in the department and converted to the allyloxy compounds on a 5.0 gram scale. The phenols were dissolved in 125 ml of distilled tetrahydrofuran (THF), deprotonated with 1.3 equivalents of potassium t-butoxide, and reacted with an excess (above 1.5 equivalents) of allyl bromide. The reaction mixture was heated to reflux, and the progress was followed by TLC with 60/40 hexane/ethyl acetate as the eluting solvent.
As the reaction progressed, a white solid (KBr) precipitated from the solution. The reaction was quenched with 100 ml of distilled water, and the THF was removed using a rotoevaporator. The aqueous layers were then extracted three times with elhyl acetate, and the extracts were dried over magnesium sulfate and evaporated. The resulting oils were purified by vacuum distillation using a bulb-to-bulb apparatus
The alloxy products were obtained in 87 to 90% yields. Each was characterized by TLC, IR, and NMR. TLC showed single spots, uncontaminated by starting material. IR spectra showed the disappearance of the -OH band at 3140 cm·1 and the appearance of several C-H bands around 3000 cm- 1. NMR data for the allylated iodophenol is typical: 4.5ppm doublet (2H), 5.2-5.5ppm quartet (2H), 6.0-6 2ppm multiplet (lH), 6.7ppm doublet (2H), 7.5ppm doublet (2H). In attempting the hydrosilation step, however, I encountered difficulty. The reaction mixture contains one milimole each of the allyloxy compound and the siloxane hydropolymer, 10 ml of specially prepared toluene, and 15 J.Ll of a catalyst solution made of 1 g choroplatinic acid, 1 g ethanol, and 98 g THF. The mixture is refluxed for 24 hours in a teflon vial, after which the toluene is evaporated with a stream of nitrogen and the crude product is analyzed by NMR. The three peaks which indicate the unreacted allyl tail are quite distinctive, and their disappearance indicates that the reaction is complete.
Three rounded peaks much further upfleld which are typical of the silicon polymers also appear.
If analysis of the crude product shows that the hydrosilation was successful, the polymer is then dissolved in dichloromethane and fractionated by adding methanol and water until there is a phase separation, shaking, centrifuging, and removing the methanol/water layer. The dichloromethane solution is then run through a pipette column of Superlig® to remove the platinum and another column of sodium sulfate (granular) to remove traces of water, and then evaporated. The resulting gum is analyzed by NMR.
My first attempts at hydrosilation was unsuccessfuL The NMR of the crude product showed only partial reduction of the allyl peaks and appearance of small polymer peaks instead of a complete reaction. I attempted it two more times with the same materials, once with a longer reaction time and again using more of the catalyst solution, with the same results.
I then began to substitute each part of the hydrosilation mixture, beginning at the easiest and least expensive. I prepared fresh toluene solvent by filtering reagent grade toluene through coconut activated charcoaL The reaction still did not go to completion. I then tried using catalyst solution from another lab, but later learned that they had merely taken some from the same bottle I was using. I then ordered more of the catalyst compound and prepared a fresh solution. The reaction gave the same results as before. I then decided to make the unsubstituted allyloxy phenol to be certain the halogen substituents were not interfering with the reaction and to avoid using up my starting materials until I was able to find the problem. The unsubstituted compound did not perform any better than the halogenated ones had.
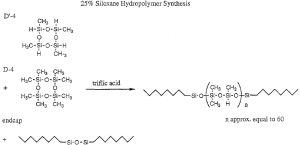
I was forced to conclude that the polymer itself was at fault. I had been using a vial of 25% siloxane hydropolymer which had been synthesized by another researcher who has graduated. NMR and IR spectra seemed to be correct, but I was told that siloxane hydropolymers may degrade over time. Therefore I set about to synthesize the polymer myself. I obtained the procedure from the researcher’s lab book. In a teflon vial are mixed 10 milimoles each of D-4 and D’ -4, 5 ~L of triflic acid, and enough endcap reagent to give polymers of molecular weights around 8000. These are stirred with a mechanical stirrer for 24 hours and then fractionated in the same manner given above. Fractionation is intended to remove unreacted materials and short chain polymers. Typical organic characterization teehnique&-such-as NMR, -IR,melting point or boiling point, carbonlhydmgen analysis, and refractive index are unfortunately ineffective in characterizing silicon hydrolpolymers; the only indication that the reaction has taken place is a thickening of the liquid.
D-4 is quite stable and is cheaply available, but D’-4 must be synthesized by a very poor yield reaction and is not easily stored. Luckily I found a small vial in the freezer. With half of it I ran the synthesis of the hydropolymer and then the hydrosilation reaction. The allyl peaks finally disappeared, but within six hours the polymer solidified into a rubbery gel, unsuitable for use as a stationary phase. I was told that this sometimes occurs if the D-4 and D’-4 are old; if they have been stored they must be freshly distilled. I distilled both using the bulb-to-bulb apparatus and used all of the remaining D’ -4 to make a batch of polymer. This time the hydrosilation was successful and the final product was a pale yellow gum.
I was finally able to complete the hydrosilation step with the four halogen-substituted allyloxy compounds. The fluoride, chloride, and bromide compounds were successful, but the iodide compound produced a reddish, rubbery substance which was insoluble in dichloromethane as the crude product. I repeated the reaction after redistilling the allyloxy phenyl iodide, which can decompose over time when exposed to light. The final product was a gum, but the NMR still showed allyl peaks. Since the other three reactions were successful I believe that the iodine atom interfered with the hydrosilation reaction.
All five polymers synthesized were and given to Dr. Milton Lee’s research group for testing over a month ago. I believed this would allow enough time to obtain results. As of this time, several gas chromatography columns have been made from the polymers, and testing has begun, but it may be several more months before they report conclusive results. Therefore I am unable to report the final results of this project. However, I am satisfied with the success of my synthetic work and problem solving. I learned a lot from the experience and appreciate the opportunity that the scholarship gave me to pursue this project.
Table of NMR Spectra
l, Starting materials
A. 4-iodophenol
B. 4-bromophenol
C. 4-chlorophenol
D, 4-fluorophenol
2. Intermediate compounds
A. 4-allyloxy-1-iodobenzene
B. 4-allyloxy-1-bromobenzene
C. 4-allyloxy-1-chlorobenzene
D. 4-allyloxy-1-fluorobenzene
3. Hydrosilation attempt (representative of many)
A. mixture of polymer and pendant group
4. Polymer synthesis
A.D’-4
B.D-4
C. endcap
D. first polymerization attempt
E. successful polymer
5. Final polymers
A. Iodobenzene-substituted
B. Bromobenzene-substituted
C. Chlorobenzene-substituted
D. Fluorobenzene-substituted