Bryon Simmons and Dr. Merritt B. Andrus, Chemistry and Biochemistry
Geldanamycin, a member of the benzoquinoid ansamycin family, was discovered in 1970 by workers at UpJohn laboratories.1 Scientists were eager to characterize and assess the biological activity of this natural product, first isolated from the bacteria species Steptomyces hygroscpius.2 It was soon learned that geldanamycin functioned as an antibiotic. More importantly, this antibiotic was also shown to check the proliferation of malignant cancer cells.3 This observation has fueled significant interest in geldanamycin as a potential antitumor drug.
In the early 1990’s, Stebbins et al.4 were able to demonstrate that geldanamycin inhibits tyrosine kinases indirectly by binding to the heat shock protein 90 (Hsp90) in the cytoplasm. Tyrosine kinases are Hsp90 substrates that play a major role in the protooncogenic signal pathway and cell cycle.
In 1995, the National Cancer Institute conducted an in vitro screening of geldanamycin against sixty of its most resistive tumor lines.4 The results were impressive. Geldanamycin was shown to be cytotoxic at doses as low as 13 nM, with a mean LD50 of 180 nM.
Encouraged by these results, Pfizer pharmaceuticals 2 began a study of the peripheral functionality of geldanamycin to determine which moieties actually participated in Hsp90- geldanamycin binding. Using a serial approach, they were able to identify exactly which functional groups interact with Hsp90 amino acid residues in the bound Hsp90–geldanamycin hydrogen bonding scheme. This work served as a starting point to understanding the binding interaction, and researchers now know the exact binding mechanism of Hsp90-geldanamycin.3
Because of geldanamycin’s novel mode of action, and the difficulty involved in obtaining pure natural samples of it, the total synthesis of the compound is now considered an important research topic. At the present date, the complete synthetic route to geldanamycin remains unknown.
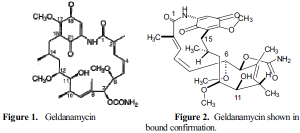
Structure of Geldanamycin
Geldanamycin consists of a planar benzoquinone embedded between a fifteen-carbon ansa ring, connected on one end by an amide linkage (Figure 1). The molecule possesses a great deal of rigidity due to the planar amide bond and three double bonds, two of which form a 1,3-diene. Bulky side groups including urethane pendant, methoxy and methyl groups also contribute to the rigidity of the molecule.2
When bound, geldanamycin adopts a much more compact conformation than that observed with the free compound. The ansa ring folds over the quinone portion forming a C-clamp-like structure (Figure 2). The internal methyl groups are positioned over the diene carbons to maximize van der Waals contacts.3
Research
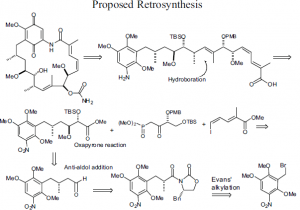
As mentioned earlier, geldanamyin has not been synthesized at present. Analogs are extracted from bacteria and modified peripherally. My research has focused on the development of a viable pathway to produce the left-hand portion of the molecule. Key reactions to reach this point were and Evans alkylation, an oxapyrone anti-aldol reaction to set the C10-C11 centers5 and a stereoselective hydroboration to set the C-8 methyl center. This has been accomplished. My future work will involve a ring closing metathesis At C8-C9 to provide a convergent synthetic methodology.
References
- DeBoer, P. A.; Wnuk, R. J.; Peterson, D. H., J. Antibiot. 1970, 9, 442-448.
- Roe, S.M.; Prodromou, C.; Obrien, R.; Ladbury, J. J. Med. Chem. 1999, 42, 260–266.
- Whitesell, L. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 8324.
- Stebbins, C. E.; Russo, A. A.; Schnieder, C.; Rosen, N. Cell. 1997, 89, 239–250.
- Andrus, M. B.; Meredith, E. L., Org. Lett. 2000, 19, 3033–3035.